Introduction
The use of CAM among cancer patients is high and is also higher than its use in the general population1-2. Most of the information on this topic has been provided by studies conducted in the United States, which show that up to 90% of these patients use complementary medicine3-5. However, there are few European studies on this topic. In 2005, a European study was conducted across 14 countries. It was found that the overall prevalence of CAM use among cancer patients was 35.9%. Spain had the fourth lowest consumption (29.8%)6.
CAM users are more likely to be younger, women, and married and to have a high educational level and annual income6-7. CAM use is more common in patients with breast, lung, and gastrointestinal cancer8,9. Dietary supplements, herbal remedies, homeopathic remedies, vitamins, and minerals are some of the most popular types of CAM used by these patients10-11. These types of products are taken by mouth and therefore could affect the therapeutic safety of patients, as suggested by studies that have found interactions between a range of substances, mainly herbs, and chemotherapy12-13.
In Spain, the prevalence of CAM use is unknown in patients diagnosed with cancer and, in particular, in those who continue treatment. This aspect is of particular interest because of the potential risk of interaction.
The main objective of this study was to determine the proportion of cancer patients who use complementary medicine while receiving intravenous chemotherapy prescribed according to usual clinical practice.
Secondary objectives were: to investigate the type and duration of CAM use, the sources of information used, the effect of CAM as perceived by patients, and to characterise the socio-demographic and clinical profile of CAM users.
Methods
Design and patients
An observational, descriptive, cross-sectional study was conducted in the ambulatory treatment unit of the reference hospital of the Autonomous Community of Navarre, Spain.
Patients referred for treatment during 2 consecutive weeks in March 2015 were invited to participate in the study. Inclusion criteria were: being at least 18 years, having a confirmed diagnosis of cancer, and receiving intravenous chemotherapy. The exclusion criterion was: language difficulties in the oral and written comprehension of the questionnaire.
The participants gave informed written consent in which they authorized access to their clinical history. They were also given an information sheet with a contact telephone number.
The variables studied were obtained through electronic medical records and a interviewer-guided questionnaire, which was completed in the treatment rooms. The study was conducted in accordance with the Declaration of Helsinki and current Spanish legislation (ministerial order SAS/3470/2009 for observational studies). The protocol was assessed by the Clinical Research Ethics Committee of the Autonomous Community of Navarre and classified by the Spanish Medicines and Health Products Agency as a “Postauthorization Study with a design other than prospective follow-up” (Spanish acronym: EPA-OD).
Questionnaire
In the absence of a validated questionnaire, one was designed following a review of the literature and subsequently assessed by an oncologist, two epidemiologists, and a clinical pharmacist (Figure 1). The questionnaire comprised 9 questions that collected the following information:
Socio-demographic data: gender, age, place of residence, marital status, and educational level.
Current CAM use: type of CAM used, beginning of CAM use (before or after cancer diagnosis), duration of CAM use, sources of information, and perceived results (no result, improvement, or adverse effects).
Based on whether the complementary medicine was taken by mouth or otherwise, the types of CAM were classified into two large groups: “Oral intake of some product” or “Other”. If the patient marked the box “Oral intake”, they were asked to identify the products as homeopathic remedies, herbs, vitamins and/or minerals, or natural remedies. Changes in diet and food were not considered as CAM, except for food marketed in the form of capsules and tablets, among others, and products from traditional Chinese medicine taken by the patient with therapeutic intent. If the patient marked the box “Other”, they were asked to identify the approach (e.g., yoga, osteopathy, acupuncture, electromagnetic fields, etc). Both sections contained a free field for further comments. The last item requested a telephone number. If authorized by the participant, the researcher could call the patient to obtain more information on the type of CAM used.
Subsequently, the clinical data of the patients were collected using the electronic medical history system of the Navarre Health Service:
Cancer diagnosis (type of tumour, stage, and time of diagnosis).
Number of cancer treatment lines received.
Surgery and/or radiotherapy as treatment.
Statistical analysis
All statistical analyses were conducted using the IBM SPSS V22.0 software package for Windows. A descriptive study was conducted using frequency and proportion analysis for qualitative variables (expressed as number and percentage), and position measurements for the quantitative variables age and duration of CAM use.
Simple logistic regression analysis was used to determine the potential predictors of CAM. We estimated the raw odds ratios (OR) and their 95% confidence intervals (95%CI).
Results
Participants
During the 2-week sampling period, 539 people attended the ambulatory cancer treatment unit for intravenous treatment. Of these patients, 58 (10.8%) did not receive treatment and therefore did not access the rooms in which the study was being conducted. A further 108 patients could not be contacted. At this point the initial sample comprised 373 patients.
However, 53 patients decided not to take part and 4 patients were excluded: 3 were excluded due to their inability to understand the questionnaire because of their lack of knowledge of the Spanish language, and 1 patient was excluded due to a psychiatric diagnosis.
Thus, the final sample comprised 316 patients (84.7% of the initial sample). Twelve patients (3.8%) were contacted by telephone to obtain further information.
Sociodemographic and clinical characteristics
The sociodemographic and clinical characteristics of the patients are shown in Table 1.
Table 1 Sociodemographic and clinical characteristics of the patients
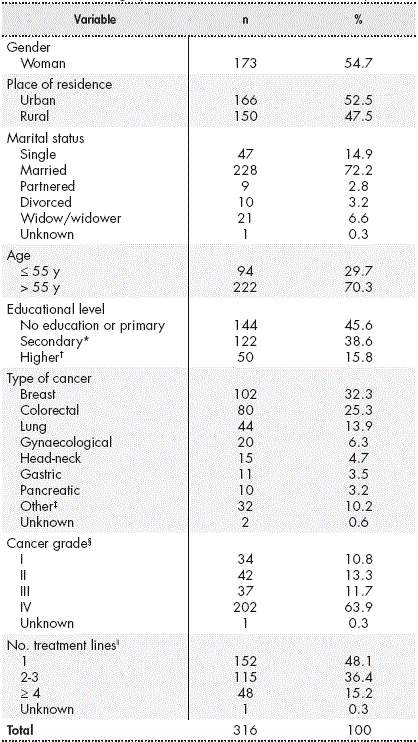
* Includes secondary education, intermediate or advanced vocational training, and baccalaureate.
† University education.
‡ Tumours with a prevalence of less than 10 patients in the study sample.
§ Cancer grade at the time of the study, based on the TNM classification of the “American Joint Committee on Cancer” (AJCC). Available at: https://cancerstaging.org/
ǁChemotherapy, immuno-oncological therapy, and hormonal therapy.
A total of 173 women (54.7%) participated in the study, and the mean age of the patients was 61 years (range, 24-85 years). Almost half of the participants lived in rural areas and almost three-quarters of the participants were married. In total, 16% had a university degree.
The most common diagnosis was breast cancer (32.3%), followed by colorectal and lung cancer. The median time elapsed since diagnosis was 12 months (range, 0-266 months). A total of 222 patients (70%) had undergone surgical intervention and 138 (44%) had received radiotherapy. Ten (3.2%) patients were participating in clinical trials at the time of their inclusion in the study.
CAM Use
The simultaneous use of CAM and conventional treatment with chemotherapy was reported by 102 patients (32.3%).
In total, 89% of the participants who used CAM took preparations by mouth. The most commonly ingested products were herbs (n = 60, 66%), followed by natural remedies (n = 35, 38.5%), vitamins/minerals (n = 32, 35.2%), and homeopathic remedies (n = 16, 17.6%).
A total of 51 different herbs were being used. The most commonly used herbs were turmeric (11.7%) followed by cat’s claw (8.3%), liquorice (8.3%), thyme (8.3%), thistle (6.7%), melissa (6.7%), and echinacea (5%). A total of 33 different natural remedies were in use. Of these, the most common were medicinal fungi used in traditional Chinese medicine (14.3%), lactobacillus (14.3%), royal jelly (11.4%), propolis (11.4%), algae such as spirulina and blue-green algae (8.6%), saccharomyces (8.6%), radish (5.7%), black garlic (5.7%), and ginger (5.7%). The most common vitamin supplements used were vitamin C (50%), followed by B vitamins (31.3%), and vitamin E (18.8%). The most common mineral salts used were zinc (21.9%), followed by magnesium (15.6%), bicarbonate (9.5%), and copper (9.4%). The most common homeopathic products used were Miracle Mineral Supplement (MMS; composition sodium chlorite, hemlock, and carcinosinum), which was taken by 43.8% of the participants who used homeopathy, and Schussler’s Salts (31.3%).
Of the total number of CAM users, 37 (36.3%) practiced “mind-body interventions”, “manipulation and body-based methods”, and/or “energy therapies”. Of these practices, the most common were yoga, reiki, the application of electromagnetic fields, salt water baths, acupuncture, hyperthermia, and relaxation exercises.
In total, 81.4% of the CAM users started to use it after cancer diagnosis (Table 2). The median duration of use was 4.5 months (range, 0-180 months).
Table 2 Answers regarding the use of Complementary Medicine
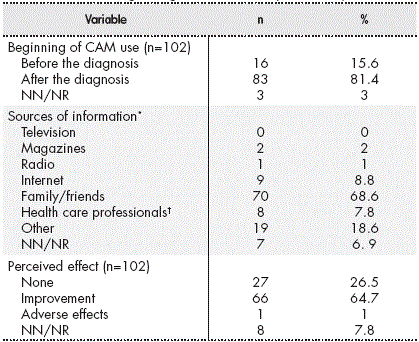
* More than 1 source of information on Complementary Medicine may have been used.
† Medical staff, pharmacists, and nurses. Abbreviations: NN/NR, not known/did not reply.
The most common sources of information about CAM were relatives or friends (Table 2). Four patients learned of CAM through a homeopath and 3 through herbalisms.
A total of 64.7% of the CAM users perceived some kind of improvement with its use (Table 2). One adverse effect was reported, which was described as stomach acidity after taking a commercial preparation of echinacea and cat’s claw. Of the patients who started using CAM after the cancer diagnosis, 52 (63%) reported that CAM was helping them in some way: 24 (29%) considered that CAM improved their physical and mental strength, 20 (24.1%) thought that it helped to alleviate the adverse effects of treatment, 14 (16.9%) believed that it helped to strengthen their immune system, and 2 (2.4%) considered that it helped them fight cancer.
Characteristics that influence CAM use
The potential predictors of CAM use in relation to sociodemographic and clinical characteristics are shown in Table 3. Female gender was positively associated with CAM use. Women were estimated to have 1.72 times (95%CI 1.06-2.80, P = 0.028) more risk of CAM use than men. Significant differences were found in CAM use by age. Taking age as a continuous variable, it was found that CAM use decreased per completed year (OR: 0.96; 95%CI 0.94-0.98; P<.001). The risk of CAM use in patients with secondary education was double that of patients with no or primary education (95%CI 1.19-3.38; P = 0.009).
Around half of the patients participating in clinical trials stated that they were using CAM at the time of the study. However, no statistical difference was found in CAM use between these patients and those not participating in trials (OR: 2.16; 95%CI 0.61-7.62).
Table 3 Socio-demographic and clinical characteristics influencing CAM use

* Raw odds ratio used to analyse the association between potential predictors and CAM use.
† The study included 315 patients who authorized access to their medical records.
‡ Includes secondary education, intermediate and advanced vocational training, and baccalaureate.
§ University education.
Abbreviations: CAM, complementary and alternative medicine.
Discussion
Little information is available on CAM use among people diagnosed with cancer in Spain. The present study provides preliminary information on the frequency of CAM use and the type of CAM used by patients receiving intravenous treatment. The justification of this study derives from the risk of interaction between CAM and conventional treatment.
The prevalence of CAM use found in this study was slightly higher than that referred by the only European multicentre study on CAM use in cancer patients for the overall Spanish population (29.8%) 6. These data are in line with those of previous studies on CAM in the field of oncology. In 1998, a systematic review of 26 studies of cancer patients in 13 countries showed that the average prevalence of CAM use was 31%14. However, due to the growing popularity of CAM, higher rates of CAM use were to be expected in the present study. In fact, CAM use has been documented in 64% to 90% of cancer patients in the United States4,5 and close to 50% in Asian countries15,16,17. This high prevalence may be related to the racial and cultural diversity of these countries, and the influence of Western and Eastern CAM practices. Studies have found high levels of CAM use in Europe, ranging from 45% to 51%9,18,19. However, an Italian study in which only current CAM use was assessed, as the present study, found a prevalence of 14%20.
Direct comparisons between the results of this study and previous studies should be made with caution, because of potential differences in sample type, sample size, methodology, and conceptualization (the definition and type of therapies considered as CAM). Due to staff shortages and time constraints, this study excluded patients being treated with oral chemotherapy, but included cancer patients receiving intravenous treatment. The special diets or juices that were included in other studies 21,22 were not included as CAM. This study only included food when it was taken as commercially capsules, tablets, and so on, or traditional Chinese medicine products15. High-protein nutritional supplements were also excluded because they form part of the conventional health care of these patients. White, green, red, and black tea were not defined as CAM, given the difficulty involved in determining whether they were used with therapeutic intent. This aspect differs from other studies, which document the use of green tea as CAM4,5,6,15. These limitations in the definition of CAM, and the fact that their current use alone was assessed, are possible causes of the lower prevalence found in the present study.
The most common type of CAM used by the participants were products taken by mouth, in contrast to practices related to the body or mind. The most commonly used substances were herbs, natural remedies, and vitamins/ minerals, as has already been documented in other studies11,19,23. This aspect reflects the attractiveness of “natural” therapies and remedies to patients, but it is precisely these substances that may involve the greatest risk6. In fact, pharmacokinetic interactions have been identified between certain herbs and natural products and chemotherapy: preclinical studies24-25 have found that garlic, ginseng, echinacea, and soy are CYP450 inhibitors. Thus, they can decrease the elimination of cytostatic drugs and increase their toxicity. In fact, garlic and echinacea were used by the study patients. Other substances used by the patients that interact with chemotherapeutic drugs were liquorice, reishi, radish, and ginger26. The most commonly used vitamin supplement was vitamin C, which is known to interact with cancer drugs such as methotrexate or imatinib27.
The majority of patients (81%) started CAM after cancer diagnosis. As found in other studies6-7, the most important sources of information on CAM were word-of-mouth or relatives and friends, whereas patients only rarely consulted health professionals. It is clear that health care professionals need to increase their awareness and knowledge of CAM use, so that they can become the point of reference in the integral treatment of the patient. The hospital pharmacist can play an important role in this process, particularly in the analysis of potential interactions between conventional and complementary therapy. It would be useful to implement alert systems that include these products in pharmaceutical validation and dispensing software.
One-quarter of the patients using CAM claimed they did not feel any improvement with CAM but continued to use it. The concept of “hope” may be a fundamental reason for the use of CAM6. The beneficial aspects of CAM use more frequently reported by the patients were similar to the main reasons for CAM use found in other studies: to obtain a good level of general health, improve physical and emotional well-being, and boost the immune system6-7,28. Although the questionnaire contained specific items on the effect of CAM, the improvements perceived by the patients could have been influenced by the effects of chemotherapy, given that both treatment modalities were used simultaneously.
Three variables were predictive of CAM use: female gender, young age, and secondary education. The first two variables have been identified as predictors by other studies6,29. Increased CAM use has also been associated with patients with higher education6,8 and with advanced stages of the disease30.
This study may have some limitations. On the one hand, patients were asked to indicate the type of CAM used by classifying it into a specific category in the questionnaire. Since it is common for patients to use more than 1 type of CAM, bias may have been present in relation to recall and knowledge of the type of CAM. In order to minimize this possibility, the patients who were unable to recall these details during the interview were contacted by telephone. A follow-up visit would have been useful to physically see the products used and thus classify them. However, due to time constraints, the researchers were unable to do so. On the other hand, the participation of two researchers in the interviews may have caused interviewer bias due to the effect that different oral and body language may have on patient responses. In order to minimize this possibility, both interviewers participated in designing the questionnaire, and agreed on the criteria for the definition of CAM and its classification.
A significant number of the patients were using CAM at the same time as their conventional medical treatment. Given that CAM is mainly taken by mouth, there is a potential risk of CAM-chemotherapy interaction. As this aspect was not an objective of the present study the magnitude of this problem remains unknown. We believe that health care professionals need to become aware of the importance of investigating CAM use among patients and to be able to advise them. This study found a statistically significant association between CAM use and female gender, younger age, and secondary education. This finding could prove useful in identifying potential CAM users. Future studies could investigate both the use of CAM in groups of patients with a specific type of cancer and potential CAM-chemotherapy interactions.
Contribution to the scientific literature
The aim of this study was to determine the prevalence of complementary medicine use among cancer patients receiving medical treatment with chemotherapy. To the best of our knowledge, this is the first study to investigate this topic in Spain. Although the prevalence of use of complementary and alternative medicine (CAM) among such patients has been documented, most of this information comes from the United States. This study shows that one- third of the patients receiving intravenous chemotherapy in ambulatory treatment units were simultaneously using other types of treatment generally taken by mouth (89%). These treatments mainly comprised herbs and natural remedies. The diversity of products was high because of the large number of ingredients included in each preparation. The high number of patients taking CAM contrasts with the low number of patients (8%) who consulted health professionals about complementary medicine. Significant predictors of CAM use were female gender, younger age, and secondary education.
Regardless of the position of health professionals toward complementary medicine, this study demonstrates that patients make use of such treatment due to the physical-emotional impact of a diagnosis of cancer and its treatment. Given the prevalence of use of complementary medicine and low number of consultations with healthcare professionals, it is clear that training in this field is needed such that the medical professional can provide advice on the effectiveness of complementary medicine and on any contraindications. The role of the hospital pharmacist is relevant during the patient interview and when reviewing possible interactions between the preparations used and chemotherapy in order to ensure its safety and efficacy.











 texto en
texto en 



