Introduction
In Spain, access to medicines in special situations (off-label use) is regulated by Royal Decree (RD) 1015/2009 dated June 19. Situations that come under this RD include the compassionate use of drugs under research, the use of medicines in situations other than authorized ones, and access to medicines not licensed in Spain. The RD states that off-label use must be exceptional, that it is typically a last resort in situations for which there is no therapeutic alternative available in Spain, and in chronic or severely debilitating diseases or those considered to threaten the life of the patient. The compassionate use of drugs under research and access to unlicensed medicines in Spain requires prior approval by the Spanish Agency of Medicines and Medical Devices (AEMPS), whereas the use of medicines under situations other than authorized ones requires local approval according to the protocol established by each hospital.
Worldwide, some 20% of drugs are used off-label, and this percentage is higher in specific populations such as pediatric and oncological patients1. Reasons for the frequent off-label use of drugs in oncology patients include the wide variety of cancer subtypes, difficulties in enrolling patients in clinical trials, the rapid diffusion of the preliminary results of drug trials, and delays in the approval of new drugs by regulatory agencies.
In 2015, the Spanish Society of Hospital Pharmacy (SEFH) published a survey on the use of off-label drugs for oncohematology patients in Spanish hospitals. The survey clearly showed that the main factor influencing the authorization-prescription process of these drugs is the available evidence. However, a lower level of evidence is usually accepted in cases in which there are no therapeutic alternatives, or in patients with low-prevalence tumors2. There is growing interest in assessing the anticipated clinical benefit of anticancer drugs3 driven by the need to optimize increasingly limited resources and provide the safest and most effective cancer therapy at the lowest possible cost. A recent study showed that a large number of anticancer drugs authorized in recent years by regulatory agencies did not provide clear clinical benefit, and that there was no relationship between the price of these drugs and their benefit to patients and society4. In addition, clinical trials typically select patients with better functional status or with specific characteristics, which calls into question their external validity.
The off-label use of drugs in oncology patients is typically based on limited evidence or on the acceptance of high costs, and thus a better understanding is required of the effects of these drugs in clinical practice.
The objective of this study was to assess the effectiveness and safety of the off-label use of oral anticancer drugs (ANEO) for cancer patients in a tertiary hospital, and to compare the results with the available evidence used to authorize the prescription of these drugs.
Method
Descriptive, observational, retrospective study. The study included all adult patients attending the Medical Oncology Service who began treatment with off-label ANEO in 2016. Patients were followed up until June 2017. Patient follow-up time was defined as the time from start of treatment to death or to the end of follow-up.
Data on the patients treated, indications, and prescribed drugs were obtained from the database of drugs in special situations recorded by the drug information center of the pharmacy department. Clinical variables were obtained from the electronic medical records (HP-HCIS®) of the hospital, and doses and duration of treatments were obtained using FARHOS® outpatient electronic assisted prescription software.
Independent variables were demographic (age, sex, functional status of the patient), treatment-related (indications, number of previous treatment lines, treatment start date, dose, schedule, change of dose or protocol, reason for change, presence of drug-drug interactions, interaction category, treatment end date), and clinical (date of disease progression, date of death). Dependent variables were survival and treatment toxicity. Overall survival (OS) was defined as the time from the start of treatment in a special situation to all-cause death or last contact with the patient.
Progression-free survival (PFS) was defined as the time from the start of treatment in a special situation to disease progression.
Toxicity was classified into several categories according to patho-physiology, anatomy, and severity using the Common Terminology Criteria for Adverse Events V3.0 (CTCAE) 5. The only adverse reactions recorded were those that caused dose modification or treatment discontinuation.
Detected interactions between ANEO and other home medications were obtained from the pharmaceutical care service and classified according to the categories defined by Lexicomp ® based on the severity of the interaction (A = no known interaction, B = no action needed, C = monitor therapy, D = consider therapy modification, X = avoid combination).
In our hospital, all off-label use of drugs needs the approval of the medical management team before the start of treatment. Although authorization for the compassionate use of a drug under research is the responsibility of the AEMPS, the request for the drug is always made with the approval of the medical management team in compliance with RD 1015/2009. Before authorizing the off-label use of a drug, the medical management team hospital liaises with the pharmacy department regarding the available evidence on its use in this special situation. The pharmacist at the drug information center provides a report on the efficacy, safety, and cost of treatment in this situation.
Evidence on the use of the requested drugs in special situations was obtained from a literature search of PubMed.
An unadjusted comparison was made between the results of the available evidence and the results of the study participants.
We calculated the median and range of the quantitative variables and the frequency distribution of the qualitative variables. Kaplan-Meier survival analysis was used to analyze survival variables. Statistical analysis was performed using the IBM SPSS Statistics® software package version 20.
Results
During 2016, treatment with off-label ANEO was requested for 44 patients, of whom 10 (22.7%) did not receive treatment due to disease progression and transfer to palliative care (n = 5), change of hospital (n = 2), enrollment in a clinical trial (n = 1), or death (n = 2). Of the 34 patients who received treatment, 50% were male and median age at start of treatment was 58 years (range, 38-80 years). The majority of the patients had grade 1 performance status as assessed using the Eastern Cooperative Oncology Group scale.
Table 1 shows the distribution of treatments and pathologies, as well as the characteristics of the patients.
Table 1 Treatments and Diseases Treated in Special Situations. Characteristics of the Patients.

ECOG, Eastern Cooperative Oncology Group.
Most of the treated patients had a diagnosis of advanced colorectal cancer and had received multiple treatment lines. At the time of the study they were receiving combination therapy with trifluridine-tipiracil.
Regarding the type of special situation, 5 of the 9 drugs requested were for compassionate use. These drugs are not yet marketed in Spain but can be purchased through the Use of Medications in Special Situations portal of the AEMPS for indications authorized in other countries by their regulatory agencies.
The other drugs used are marketed in Spain, but were used for an indication not included in their Summary of Product Characteristics.
Regarding the effectiveness of the treatments, Table 2 shows survival results compared with the clinical evidence used for the authorization of treatment6-14.
Table 2 Efficacy Results Obtained in Clinical Trials and Those Obtained in our Experience at the Hospital. Date of Analysis, June 2017.
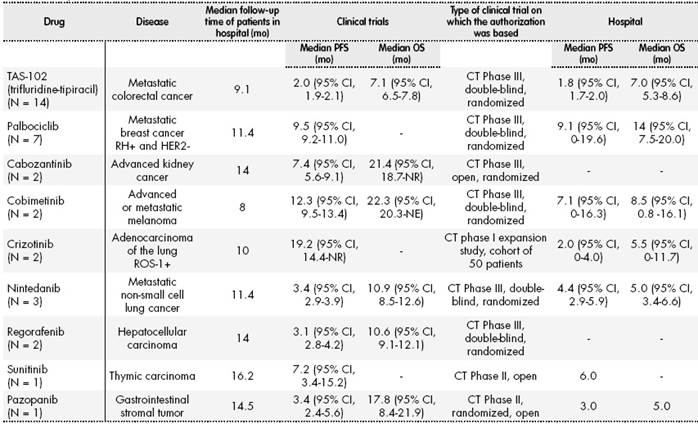
CT, clinical trial; NR, not reached; OS, overall survival; PFS, progression-free survival.
The PFS and OS rates obtained with trifluridine-tipiracil were similar in our study and in clinical trials. In the case of palbociclib, the PFS rate in our study was also similar to that in clinical trials; however, the OS rates cannot yet be compared because the data are still immature.
In the case of treatment with cabozantinib, there had been no change in PFS after a median of 14 months of follow-up, so comparisons cannot yet be made.
There were marked differences between the results of crizotinib use in our study and those of published cohort studies, even though the 2 patients receiving this drug in our study were relatively young and with good functional status, one of whom was receiving first-line treatment. One patient died within a week of starting treatment, and the disease progressed after 4 months in the other patient, who is currently under treatment with lorlatinib.
Of the 2 patients treated with cobimetinib, 1 was changed to immunotherapy with nivolumab, and the other continued treatment combined with vemurafenib.
Of the 3 patients treated nintedanib, 2 continued treatment and the other died. Comparisons cannot be made because the data are still immature.
For all patients, median PFS was 2.8 months (95% confidence interval (CI), 0.8-4.8) and median OS was 8 months (95% CI, 3.4-12.5).
Regarding treatment safety, 26% of the patients (n = 9) required dose reduction due to treatment toxicity associated with 5 of the drugs (cabozantinib, nintedanib, sunitinib, regorafenib, and trifluridine-tipiracil). Regorafenib was associated with the majority of adverse reactions. The most common of these was asthenia (33%) followed by hand-foot syndrome (22%). Table 3 shows the adverse reactions requiring dose reductions. No treatment was discontinued because of its adverse effects.
Table 3 Drugs That Needed Dose Reduction Due to Adverse Reactions. Description and Frequency of Adverse Reactions.
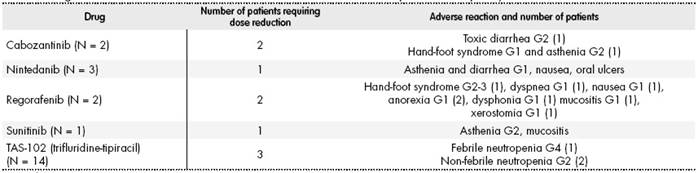
G, Grade.
We observed 13 drug-drug interactions, which affected 15 patients (44.4% of the total). Only 2 interactions were category X (avoid combination): these were palbociclib-metamizole (in 2 patients, 5.8%) and cobimetinib-carbamazepine (1 patient, 2.9%). Two interactions were category D (consider therapy modification): cobimetinib-bromazepam (1 patient) and pazopanib-escitalopram (1 patient). The remaining drug-drug interactions were category C (7 interactions in 8 patients) and category B (2 interactions in 2 patients).
In the case of category X interactions, it was recommended to replace metamizole with another analgesic that did not interact with palbociclib. In the patient receiving cobimetinib-carbamazepine, close monitoring of blood carbamazepine levels was recommended because the drug was needed to control epileptic seizures.
Discussion
A comparison of the number of patients for whom treatment in special situations was requested and the number who received treatment shows that 22% did not start the treatment, which was generally due to transfer to palliative care or death. Thus, a large percentage of these patients were at end of life or receiving palliative care.
However, in general, the patients receiving off-label ANEO were young and most of them had good functional status.
Median PFS was almost 3 months, whereas OS was 8 months. These results are indicative of anticipated survival times in patients with advanced disease who receive treatment, like the study patients, although these results should be interpreted with caution due to the heterogeneity of the diseases. The survival results are similar to those of other Spanish studies on the off-label use of drugs in oncology15, such as the study conducted by Arroyo Alvarez et al., which reported a median PFS of 5 months and a median OS of 11 months.
The main limitation of the present study is the short follow-up period; thus, some of the data are still too immature for their analysis, especially in cases in which longer survival times have been described, such as those observed with cobimetinib associated with vemurafenib or palbociclib. Another limitation is that the comparison of the results obtained from clinical trials and those obtained in our study was not adjusted and should be interpreted with caution.
In our study, trifluridine-tipiracil was the most commonly used off-label drug, and was associated with the greatest number of adverse events in our patients, with a median PFS and median OS similar to those of clinical trials. Thus, the data show that this treatment provides very marginal survival gains in patients with heavily pretreated colorectal cancer.
As described in other studies, asthenia was the most common adverse event, and regorafenib was associated with the greatest range of adverse events.
As noted in the SEFH report on the off-label use of anticancer drugs2, the low prevalence of some tumors or the lack of alternatives can lead to the authorization of treatments with a very low level of evidence on their effectiveness. In countries such as Italy, the reimbursement of off-label anticancer drugs in some cases depends on the results of therapy in real life, especially when there is a lack of evidence prior to its use16, following an individualized payment-by-results approach for each patient.
Follow-up of the results of off-label drug use in our hospital is vitally important because the results of their use in clinical practice should be used to assess the authorization of future treatments at the hospital. Likewise, the implementation of a pharmaceutical care service for cancer patients at our hospital has allowed us to closely monitor the effectiveness and safety of such treatments in each patient, thus preventing the prolongation of ineffective or unsafe treatments and allowing us to optimize the available resources.
The effectiveness of off-label ANEO in our hospital is similar to the evidence available from clinical trials. Their impact on survival is limited and adverse effects are common. The pharmacy department should participate in the authorization process, pharmacotherapy follow-up of the patient, and follow-up of the results of these therapies. This information should be taken into account in future decision making.
Contribution to the scientific literatura
This study presents data on the effectiveness and safety of oral anti-cancer drugs used in special situations (off-label use), and compares the results with the evidence used for their authorization.
In the setting of palliative treatment, the results show that these drugs have little impact on survival and have a high rate of adverse effects. This information may be of assistance in future decision-making in this type of setting in the future.











 text in
text in 


