Introduction
High-risk medications (HRM) are medications that incur a high risk of causing severe or even fatal harm to patients when used incorrectly1,2. The Institute for Safe Medication Practices (ISMP) published a list of drugs considered to be high risk in hospitals3,4. This list includes intravenous vasoactive drugs (VAD), such as adrenergic agonists, dopaminergic agents, and organic nitrates. The inclusion of medications in this list does not mean that errors associated with these medications are more frequent, but that if they were to occur, the consequences could be more severe1. For this reason, HRMs are a priority for professional bodies concerned with patient safety.
No single practice can guarantee complete safety when working with HRMs. Thus, in order to reduce preparation errors, we recommend the implementation of different specific practices, such as the use of detailed and explicit protocols, and the centralization of the preparation of intravenous mixtures of HRMs in the Pharmacy Department (PD)1.
The concept of the Intravenous Mixtures Unit (IVMU) appeared in the 1960s as a way to guarantee the stability and compatibility of intravenous mixtures (IVM). However, the centralized preparation of IVMs other than parenteral nutrition and chemotherapeutic agents is common in the USA, but not in Europe. In Spain, the preparation of IVMs that require the handler to be protected is widely accepted. Compared to the rest of Europe, the situation is better in Spain5,6, where 9.5% of PDs prepare most of the IVMs.
The preparation of IVMs in hospital care units involves a range of factors that can generate errors. In addition, the preparation of different concentrations increases the risk of error7,8,9. For these reasons, an IVMU is a key asset in the prevention of medication errors10,11. Furthermore, the centralization of the preparation of intravenous mixtures of HRMs enables their standardization1, as corroborated by the Guide to Good Practices for the Preparation of Drugs in Hospital Pharmacy Departments12.
Although there are still few studies on the costs of preparation of IVMs in the PD compared to their preparation in hospital care units, it has been suggested that the batch preparation of IVMs in an IVMU may be more cost effective than their preparation in hospital care units13,14,15.
The centralization of the preparation of intravenous VADs in our hospital led to improved patient safety16. Based on this result, we conducted a time-cost analysis to assess the efficiency of the process.
The main objective of this study was to evaluate the efficiency of protocolization and centralization of the preparation of intravenous VADs in the treatment of critically ill patients. The specific aims were:
To analyse the preparation times of vasoactive IVMs with or without protocolization and centralization in the PD.
To assess the economic impact of the protocolization and centralization of the preparation of vasoactive IVMs on costs in the treatment of critically ill patients.
Methods
A prospective interventional study was conducted in a tertiary hospital (July 2012-December 2014) to measure the impact of the implementation of vasoactive IVM protocols on safety, efficacy, and costs in the treatment of critically ill patients16. Firstly, a working group was set up to select the drugs and preparations to undergo protocolization and standardization. Secondly, the group organized the centralization of the preparation of protocolized IVMs in the PD. The preparation of IVMs in the PD was sequentially implemented in the following Intensive Care Units (ICU): General Medicine (10 beds), General and Digestive Surgery (10 beds), Cardiovascular Surgery (10 beds), and Coronary Care Unit (13 beds).
From these ICU units, we selected patients ≥18 years receiving treatment with 1 or more of the protocolized vasoactive IVMs. Patients participating in clinical trials were excluded, as well as patients from the neurosurgical ICU, because of the lack of an electronic prescription system in that unit.
Results on safety and efficacy have already been published16. To perform the cost analysis, the following variables were measured:
IVM preparation time: time in minutes of the complete process of the preparation of an IVM in the hospitalization unit or in the PD. The complete process included: time of preparation of materials; and preparation of the IVM and labelling.
To determine the time of preparation of an IVM in the PD and in the hospital care unit, we recorded the time needed to complete all the tasks in the preparation process: verification of the IVM to be prepared; preparation of the materials; preparation of the IVM; disinfection of materials and work surface; and labelling. The pharmaceutical validation of prescriptions was assumed to be similar in both settings.
A. Pharmacy Department:
Pharmaceutical time necessary to establish Standard Operating Procedures (SOP) relying on the data available in the Product Catalogue and Invoicing of the Spanish Society of Hospital Pharmacy (SEFH)17 for each new IVM, and time spent in training staff.
Creation of worksheets and labels. Subsequent verification (pharmacist).
Preparation of the medication and packaging materials (pharmacy assistant).
Disinfection of materials and work surface, and preparation of the IVM (Batchelor of Science in Nursing (BSN)).
Labelling (BSN).
Final check of the prepared IVM using the worksheet (BSN).
B. Nursing control:
Using of the nursing checklist to confirm the prescribed IVM and the guidelines to follow in its preparation (BSN).
Preparation of the medication and packaging material (BSN).
Disinfection of the work surface and preparation of the IVM (BSN).
Preparation of the label and labelling of the IVM (BSN).
Some of the preliminary process times (clarification of questions, pharmacy refrigerator checks, calculations needed for scheduling, etc) were not taken into account, because some of these actions were not performed in the hospital care unit. Rest breaks and hospital porter times were also not included, because IVMs were distributed from the PD to the clinical units with other medications while refilling the Automated Dispensing Systems (ADS).
In the PD, the time needed to prepare the batches of IVMs was recorded over 8 working days (405 MIV in total). In the hospital care unit, 2 experienced nurses from 2 different ICUs prepared 3 different IVMs per day for 5 consecutive working days (30 MIV in total). Taking into account the daily batch preparation of the new IVMs in the PD, and individualized preparation per patient in the ICUs according to usual practice, we estimated that the samples would be adequate to perform the analysis6. Process times were recorded by the research pharmacist.
Preparation costs: final price of an IVM in 2014 Euros (€). Costs included:
Cost of medication and diluent (Average Invoicing Price (AIP)): net cost, including 4% VAT and applicable discounts (€).
Staff costs: gross salary of healthcare staff (€).
Medical supplies costs: net cost, including VAT (10% or 21% according to the article) and applicable discounts (€).
Costs associated with the use of laminar flow cabinets (LFC).
To assess the impact of the protocolization on the cost of treatment, we compared direct costs (fixed and variable) of the preparation of vasoactive IVMs in the PD and in the hospital care unit. The cost of the facilities and the purchase cost of the cabinets were not taken into account because they were already amortized. The cost of maintaining the area was not included in the calculations. To establish the LFC operating, maintenance, and annual filter change costs, we calculated the percentage of the LFC operating time dedicated to the production of IVMs per week.
Net costs of drugs and diluents were obtained from the PD management software database. The cost of medical supplies was obtained from the Supply Service. Staff costs were obtained from the Official Bulletin of the Community of Madrid of January 31, 201418, taking into account the total annual salary in the categories BSN and Medical Specialist. Table 1 shows gross staff salaries and net medical materials and supplies costs.
Table 1 Unit Costs Used in the Analysis (2014 Euros)
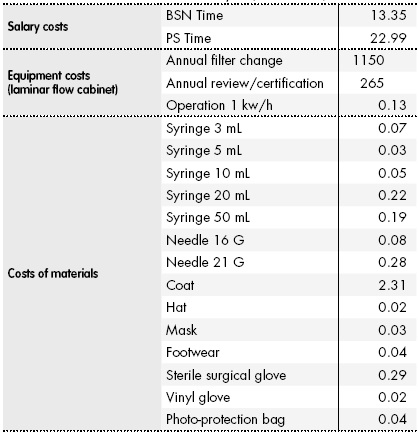
BSN, Bachelor of Science in Nursing; PS, Pharmaceutical Specialist.
To determine the final cost of preparation of IVMs, we included medication, diluent, medical supplies, staff costs, and use of LFC.
We also calculated the cost of unused and expired IVMs in the PD or in the ADS of the hospital care unit. The cost of disposal of expired IVMs was not included.
Finally, we conducted a univariate deterministic sensitivity analysis to assess whether the results obtained in the baseline analysis were sensitive to changes in the main variables susceptible to uncertainty or with a certain margin of variability. Thus, the following scenarios were considered:
- Exclusion of the cost of expired IVMs. We also estimated to what degree preparation could be optimized to achieve a reduction in the total number of expired IVMs per year.
- Exclusion of the time-cost of the pharmacist required to create new SOPs. To facilitate the analysis, a spreadsheet was designed using Excel 2007.
All estimates were made for a 95% confidence interval (95%CI), and a P value of <.05 was used as a cutoff for statistical significance. Time and cost variables were expressed as mean and standard deviation. The symmetry of the variables was examined using graphic methods and the Shapiro-Wilk normality test. Means were compared using the Student t test for independent samples using the Stata software package version 12.0.
Results
IVM preparation time
In the IVMU, 18 batches were prepared (average 23 ± 12 units/batch) with a mean IVM preparation time of 2.10 ± 0.77 minutes (95%CI, 1.33 - 2.87).
In the hospital care units, the mean IVM preparation time was 2.86 ± 1.22 minutes (95%CI, 1.64 - 4.08).
The difference in preparation times (2.10 vs 2.86 minutes) was statistically significant (P = 0.002), showing that preparation in the IVMU was more efficient. Preparation time in the IVMU was 26.41% lower than preparation time in the hospital care unit.
In 2014, 8433 IVMs were prepared in the IVMU. The mean time gained by preparation in the IVMU was 0.76 min/IVM (1.26 h/100 IVM (95%CI 0.50 - 2.00) and 106.26 h/y (95%CI, 42.02 - 169.92)).
Preparation time was lower in the IVMU for all types of IVM, except for adrenaline; however, the only difference in preparation time that reached statistical significance was for noradrenaline (p = 0.007).
Cost of IVM preparation
Table 2 and Table 3 show the cost of medicines, saline solutions, and medical supplies, and the cost of preparation in the hospital care unit and in the PD. The unit cost per IVM was lower in the IVMU for all IVMs.
Table 2 Processing Costs in the Hospital Care Unit (2014 Euros)

GS, 5% glucose solution; IVM, intravenous mixture.
Table 3 Processing Costs in the Pharmacy Service (2014 Euros)

GS, 5% glucose solution; IVM, intravenous mixture.
IVM preparation required 58.82% of the operating time of an LFC working surface, entailing filter change costs (€676.47), annual maintenance costs (€155.88), and operating costs (€34.58). Taking into account the total number of IVMs prepared per year, the additional cost of using the LFC was €0.10 per IVM.
The establishment of protocols for each new IVM prepared in the PD needed 180 minutes of pharmaceutical time (5 new SOPs = 15 hours in total)17. Taking into account the total number of IVMs prepared per year, the additional cost in pharmacist time was €0.04 per IVM.
Table 4 shows the final cost of each type of IVM by hospital care unit and PD. The average cost per IVM was €5.24 ± 1.45 in the PD and €5.62 ± 1.55 in the hospital care unit, entailing a saving of 6.76% in the IVMU. The difference did not reach statistical significance (P = 0.701).
Table 4 Final Cost of Preparation in the Pharmacy Department vs the Hospital Care Unit (2014 Euros)
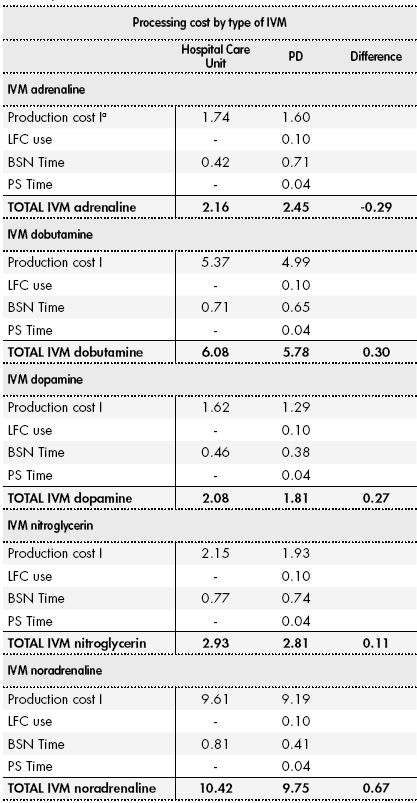
aProcessing cost I includes medication, saline solution, and materials. BSN, Bachelor of Science in Nursing; CFL, laminar flow cabinet; PS, Pharmaceutical Specialist.
Given that 8,433 MIVs were prepared in 2014, the overall cost of preparation was €44,188.92 in the PD and €47,393.46 in the hospital care unit. However, the inclusion in the analysis of IVMs that reached their expiry date before use showed that centralized preparation in the PD was more expensive than preparation in the hospital care unit (€2174/y).
Sensitivity analysis
Scenario 1: Exclusion of the cost of prepared IVMs that reached their expiration date before use. In this scenario, the centralized preparation of IVMs in the IVMU entails a potential saving of €3165/y.
We also considered the optimization of IVM production, and assessed the impact of halving the total number of expired IVMs. In this case, the difference in costs, although lower, would still be favourable to centralized production with savings of €449/y.
Scenario 2: Exclusion of pharmacy time needed to design the new SOPs. The average cost of preparation per IVM would be €5.20 ± 1.44 in the PD and €5.62 ± 1.55 in the hospital care unit. However, the overall cost difference would not be favourable to centralized preparation in the PD (€1921 more per year).
Combining the 2 scenarios showed that centralized preparation would entail a potential saving of €3511 per year, although this result did not reach statistical significance.
Discussion
The difference between preparation time in the PD and preparation time in the hospital care unit was favourable to centralization in the PD. However, in the hospital care unit, the preparation time of adrenaline was shorter, which could be because the new standard dilution (1.6 mg/100 mL) uses only part of the content of 1 ampoule rather than the full unit. The use of part units could increase preparation time in the IVMU, because only the use of whole units would make it possible to systematize the procedure.
The activities included in the measurement of IVM preparation times are not homogeneous in the literature, which makes it difficult to compare results. In this study, the preparation times in the PD and in the hospital care unit were slightly longer than those reported in other studies6,19. However, the times were shorter than those reported in other studies that used a similar methodology13,14, although these studies included preliminary activities not included in the present study (medical order transcription, label printing, double checking by the nursing staff). Nonetheless, our results are supported by the overall similarity between the methodology used in the present study and that used in these other studies (method used to measure time, similar preparation process in the PD and in the hospital care unit, the specific definition of all the activities included in the process).
It should be noted that in one of these studies19, a pharmacy technician prepared most of the medications prepared in the IVMU. However, in our IVMU, BSNs prepared the medications, which should be taken into account when comparing staff costs. Increasing the number of technical staff would reduce staff costs in the PD. Nevertheless, the main cause of the reduction in preparation times in the PD was centralized preparation, which permits batch production instead of individual dose production. Every 100 IVMs prepared in the IVMU saves nursing time, which could be used to greater effect in the hospitalization unit to improve patient care. Therefore, centralization is an opportunity to improve efficiency in the use of medication12 and in the use of human resources, which in itself could be considered a means to contain costs6.
Although the overall cost analysis showed that centralized preparation was not more economical than traditional preparation when the cost of expired IVMs was included in the analysis, we consider that the marginal increase in costs is acceptable given the marked improvements in safety, since there was a significant decrease in prescription errors (55.89%), validation errors (68.05%), and administration record errors (78.75%)16. Some studies have found an association between reduced costs and avoided errors. In Spain, a study conducted in a tertiary hospital used the calculated cost of an adverse event in the United States, concluding that medication errors caused an average increase of 303 days in hospitalization time, with an annual increase in costs of approximately €76,00020.
We believe that the result “not favourable” to centralization may be due to the low cost of the drugs studied. In fact, other studies conducted with antibiotics, antifungal agents, and drugs with greater economic impact have shown savings following centralization6,19,21,22. Furthermore, the present study did not take into account savings in drug costs that would have been generated by the use of multidose vials6. It was not possible to assess this scenario using the specialty pharmaceuticals marketed for the active ingredients studied. If an increase in the number of IVMs prepared in our IVMU was under consideration, we would recommend the use of multidose vials. Savings in the IVMU could be increased by the use of large-volume saline solution for the reconstitution of lyophilized specialty pharmaceuticals. Furthermore, centralization would lead to cost savings in medical supplies. For example, the centralized preparation of a batch of medication uses 1 syringe and needle, whereas the preparation of each IVM in the hospital care unit uses 1 syringe and needle. This study found that the costs of medical supplies were 3.4 times higher in the hospital care unit, which result is in line with those of other studies13,14.
Sensitivity analyses showed that centralization always entails a potential saving if the IVMU optimizes its production by adjusting stock according to need, thus reducing the number of IVMs that unnecessarily expire before use. This aspect particularly applies to the case of noradrenaline, which is the most expensive and less stable IVM16. This approach would also avoid stock shortages, which would lead to higher costs in the preparation of IVMs in hospital care units. This point should be confirmed by further studies. Likewise, the implementation of protocols would eliminate pharmacy time costs in the creation of new SOPs, and thus these costs would not affect the average cost of preparation per IVM in subsequent years.
Although the use of multivariate probabilistic sensitivity analysis is generally recommended for this type of study, some aspects, such as the effect of changing some assumptions, can only be assessed using deterministic sensitivity analysis. Despite its limitations, we consider that the univariate deterministic sensitivity analysis used in this study was adequate to meet its objectives23,24.
Another limitation is that the measurement of preparation time was not blinded, which could have influenced the results. However, the Hawthorne effect (i.e., improvement in the activity of a worker when monitored), gradually disappears over time25,26. Moreover, this effect would have equally affected measurements in both scenarios in the present study.
In conclusion, the analysis showed that there was a significant reduction in the preparation time of vasoactive IVMs used in the treatment of critically ill adult patients when they are prepared in the PD in comparison with their preparation in hospital care units. The difference in costs of preparation in the PD, mainly caused by expired IVMs, would be eliminated by optimizing production in the IVMU and minimizing losses due to expired IVMs.
Contribution to the scientific literature
This article presents an economic analysis of a previous study conducted in the setting of Hospital Pharmacy to assess the impact of the protocolization and centralization of the preparation of intravenous vasoactive drug mixtures in critical care patients. Specifically, we assessed the efficiency (time and cost) of the centralized preparation of different intravenous vasoactive drug mixtures (IVM) in the Intravenous Mixing Unit (IVMU) of the Pharmacy Department.
Given the importance of the centralized preparation of drugs, as corroborated by the Guide to Good Practices for the Preparation of Drugs in Hospital Pharmacy Departments, we consider the topic of this article to be of interest to our fellow professionals. It should also be taken into account that there has been an increase in the workload of IVMUs due to this task, and thus this study is also motivated by the need to support this activity by providing time-cost analyses.











 texto em
texto em 


