Introduction
Currently, the pharmaceutical industry does not offer forms that cover all the needs of ophthalmological treatment. Thus, this therapeutic gap should be addressed by the centralised preparation of such forms in hospital Pharmacy Services (PS) using authorized medications for other indications or administration routes.
The use of medicines under conditions other than those described in the summary of product characteristics is regulated by the Spanish Royal Decree (RD) 1015/20091, which defines this approach as “The use of medicines under conditions other than those authorized” (Spanish acronym: UMCDA). Chapter III, article 13 of the RD establishes the requirements for such use and includes the following statement:
The doctor must provide a sound justification in the clinical history of the need to use the medication.
The responsible doctor must obtain the consent of the patient according to Spanish Law 41/2002 on patient autonomy.
Suspected adverse reactions must be notified according to the provisions of RD 1344/2007 regulating the Pharmacovigilance of Medicinal Products for Human Use.
Restrictions that have been established related to the prescription and dispensation of the medication and the therapeutic protocol of the centre will be respected.
The fact that the UMCDA may be linked to a protocol prepared by the health centre serving the patient involves the need to carefully assess the available evidence on each medication for each of the indications for which it is intended.
Furthermore, in 2011, in resolution CM/ResAP (2011)12, the Council of Europe aimed to standardise the quality of medicinal products, and recommended the development of practical guidelines on drug preparation to avoid differences in quality and safety between medications prepared in pharmacies and those manufactured on an industrial scale. Thus, in 2012, in its adaptation to Spanish regulations, RD Law 16/2012 was published on urgent measures to guarantee the sustainability of the National Health System and improve the quality and safety of its services3. RD Law 16/2012, article 7 establishes that the PSs in which these operations are conducted must guarantee adherence to the good practice technical guidelines. Therefore, the preparation of medicines must meet the quality criteria outlined in the Good Practice Guidelines on Pharmaceutical Preparation (Spanish acronym: GBPP)4 in order for them to be dispensed in a form ready to be administered under the required conditions after risk assessment and assignment of the necessary quality criteria. The preparation of prefilled syringes ready to use is included as one of the operations of the manipulation and adaptation of preparations 3. Specifically, the division of very high-cost therapeutic substances from a commercial single-use vial into multiple individual doses is frequently performed by hospital PSs in the attempt to minimize the high economic impact of these therapies.
Based on the risk assessment, preparations for intraocular administration should be prepared centrally in PSs in a laminar flow cabinet (LFC) with a controlled environment given the possibility of patients experiencing adverse effects caused by the contamination of these types of preparations4. However, although there are many general recommendations for the preparation of these types of products, to date there is no unanimous consensus on specific techniques to maximize benefits, while obtaining the largest number of individual syringes possible and ensuring the sterility, stability, and effectiveness of the doses prepared.
The objectives of this document are: (1) to establish which drugs and under what conditions there is sufficient pharmaceutical and clinical evidence to support their use other than that described in the summary of product characteristics in order to cover the most common therapeutic gaps in ophthalmological treatment, and thus facilitate the development of care protocols in health centres; and (2) to establish a set of general recommendations for the preparation of intraocular injections that are useful for the health personnel involved in their preparation and that increase patient safety.
Methods
This document was developed by a working group comprising members of the Spanish Group of Pharmaceutical Compounding of the Spanish Society of Hospital Pharmacy (Spanish acronym: SEFH) and members appointed by the board of the Spanish Society of Ophthalmology (Spanish acronym: SEO).
The document was developed in several phases:
The members of the Spanish group of Hospital Pharmacy Compounding shared information on the ophthalmological medications they most frequently prepared in their own health centres, their indications, and the galenic and clinical evidence on which they were based.
After drawing up an initial list, it was reviewed by the SEO assessment group for possible corrections or the inclusion of new preparations.
The list reviewed by the SEO underwent a final literature search of the galenic evidence related to the selected preparations using PubMed. Thus, the following keywords were used: ophthalmic solutions, drug stability, keratitis, endophthalmitis therapy, intravitreal injections, intraocular injections, and drug compounding.
Members of the Spanish Group of Hospital Pharmacy Compounding used the GBPP4 as a reference to establish recommendations on the preparation of intraocular injections. This group also used PubMed and other electronic literature sources to conduct a literature search using the following keywords: intraocular injections, intravitreal injections, pharmaceutical preparations, drug compounding, drug stability. In this way, a draft recommendation was created on the characteristics of the environment, material required, preparation technique, shelf-life, and quality control of the intraocular preparations. This draft was shared among the members for its review and for further contributions taking into account the expertise of each professional in each of the sections mentioned. In this phase, it was decided to include aspects related to syringe packaging, prescription, and traceability of the samples.
• Finally, the document was reviewed and endorsed by the SEO assessment group within the framework of the SEO-SEFH collaboration agreement. The document was signed by the board of the SEO on November 16, 2017.
Results
Recommendations on the use of ophthalmic medications
As mentioned, the use of medications under conditions other than those authorized involves respecting the established restrictions on the prescription and dispensation of the medications and following the health care protocol of the centre. This protocol (or protocols if it has been decided to design one for each active ingredient) must be agreed upon by the services involved in the use of the medication and approved by the Pharmacy and Therapeutics Commission and by the management of the health centre.
Table 1 shows a proposed protocol for the use of ophthalmic drugs under conditions other than those authorized.
Table 1 Proposed protocol for the use of ophthalmic drugs under conditions other than those authorized

In each application the prescribing physician provides a sound justification in the clinical history of the need to use the medication, inform the patient of the potential benefits and risks, and obtain their informed consent.
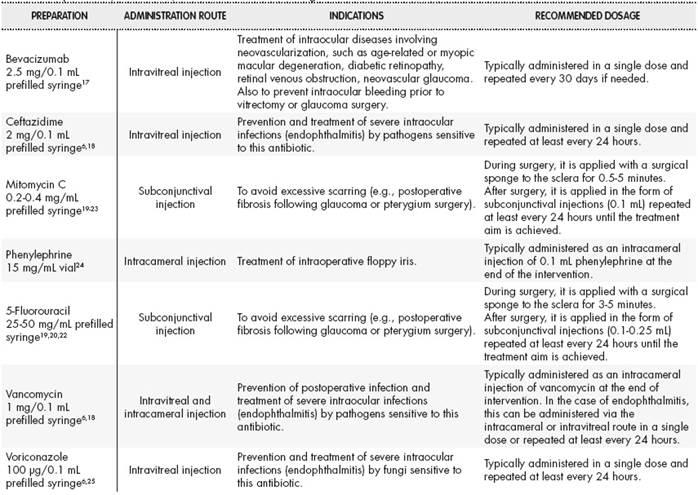
Table 2 and Table 3 provide examples of eye drops and intraocular injections, respectively, their indications (unauthorized), and the most common posology.
General recommendations on the preparation of intraocular preparations
Annex 1 and Annex 1(cont.) shows the recommendations for the preparation of intraocular preparations. This annex provides information on the following aspects: preparation area, characteristics of the material, preparation technique, packaging, shelf-life, quality control, prescription, and traceability.
Depending on the type of preparation, special requirements will be taken into account for the preparation of terminal sterilization products or for aseptic preparation as outlined in the GBPP4.
Conclusions
There is ample clinical evidence supporting the use of the drugs selected in this study for ophthalmic use, although the health authorities do not recognize such use in the majority of cases.
From a legal point of view, these preparations would be included in what is known as the use of medicines under conditions other than those authorized. Their use should be incorporated in the health care protocols agreed by the ophthalmology services and approved by the Pharmacy and Therapeutics Commission and the centre’s management body.
There is also galenic evidence supporting the preparation of these medications such that they can be administered by the ophthalmic route in the form of eye drops or intraocular injections.
Ophthalmic medications must be prepared in accordance with the guidelines established in the GBPP. In addition, the specific recommendations on intraocular preparations established in this document will help to standardize and facilitate the preparation of these medications in hospital PSs, thereby making these types of preparations equally accessible to all patients.











 texto en
texto en