Introduction
Human immunodeficiency virus (HIV) infection is now considered to be a chronic disease thanks to the striking decrease in mortality following the introduction of highly active antiretroviral therapy (HAART) and the subsequent arrival of new more potent drugs with better dosage regimens1. Increased survival has led to the parallel ageing of the HIV-positive population. In fact, data on different cohorts of HIV-positive patients have already been published in international studies, showing that more than 50% of the individuals in treatment were already more than 50 years old. It has been suggested that by 2030 this percentage will have increased2.
The aging of the population has led to an increase in a range of comorbidities. It is also known that these comorbidities have a greater prevalence and occur earlier in HIV-positive patients than in the general population. It has been estimated that aging will accelerate over the next 10 years3-6. Simultaneous with the increase in comorbidity, the concept of polypharmacy has gained increasing exposure in the setting of the increased prevalence of HIV-positive patients7, although, according to most authors, there is no consensus on its definition8. With the aim of providing a more accurate picture of pharmacotherapy, rather than a simply quantitative one, the term medication regimen complexity has been coined. Different tools are available for its measurement, and its relationship with different health outcomes is already known9-12.
The experience and scientific evidence obtained with the general population are already serving as a starting point for the assessment and management of elderly patients with HIV13-16. However, knowledge and consensus on many key factors is needed within the multidisciplinary teams caring for these patients, which would help to reorientate and improve healthcare in this population.
The objective of this review was to describe the current state of knowledge and the management of aging and medication regimen complexity in HIV-positive patients.
Methods
A literature search was conducted using the following databases: Medline (via Pubmed), Scopus, Web of Science, the Cochrane Library, and Google Scholar.
Inclusion criteria were: all English-or Spanish-language original or review articles published between 2007 and 2017 which analysed the management of aging and medication regimen complexity in HIV-positive patients older than 50 years.
The Medline database was searched using Medical Subject Headings (MeSH), which is a thesaurus created by the US National Library of Medicine. The following English and Spanish terms were used in combination: “Polypharmacy”/“Polifarmacia”, “Aging”/“Envejecimiento”, “Frailty”/“Fragilidad”, “Complejidad Farmacoterapeutica”/“Medication Regimen Complexity”, and “HIV”/”VIH”.
The articles were independently reviewed by two of the authors of this article. Disagreements were resolved by consensus. Inter-rater agreement on the inclusion of the selected articles was assessed using the Kappa index (a cutoff value of 0.80 was used to indicate good correlation).
Subsequently, we analysed and combined the data extracted from the articles that met the inclusion criteria.
Results
A total of 208 bibliographic references related to the study objectives were identified and analysed. Figure 1 shows the flowchart of the revised bibliography according to the inclusion and exclusion criteria used. Table 1-1 and Table 1-2 shows the selected studies, which include data relevant to the setting of HIV-positive patients.
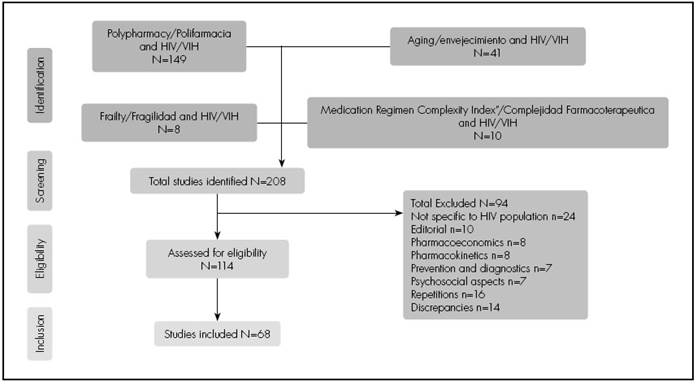
Figure 1. Flow Diagram of the Literature Review and Inclusion/Exclusion Process of the Studies Analysed.
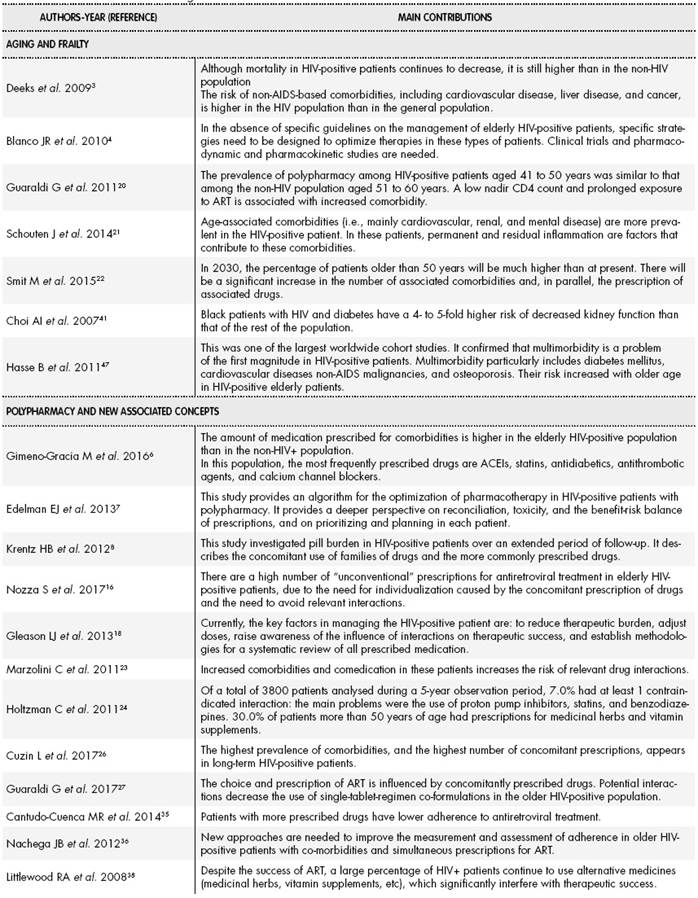
In general, polypharmacy has been defined as the concomitant and simultaneous use of multiple medications. However, the term has many definitions, which include aspects such as the use of potentially inappropriate medications (PIMs), the underuse of medication, or therapeutic duplication. Different numerical cutoff points have been used in published studies, although most have defined polypharmacy as the use of 5 concomitantly prescribed drugs17,18. However, since the publication of the document on HIV and advanced age by the National AIDS Plan and the Spanish Society of Geriatrics and Gerontology in 2015, which included this definition, a consensus has emerged in Spain that patients are polymedicated if they simultaneously use 6 or more active principles. This definition will be used to correlate and compare future studies19.
Different studies have already demonstrated the relevance of the problem from a qualitative perspective. Guaraldi et al. (2011) were the first authors to show that more than 2 chronic comorbidities were more common in HIV-positive patients older than 50 years than in non-HIV-infected participants of the same age20. These data were confirmed by Schouten et al. in 201421. Furthermore, a modelling study by Smit et al. suggested that 84% of HIV-infected patients would have more than 2 comorbidities by 203022. Marzolini et al. published the first study to show that 50 years is the age at which HIV-positive patients need significantly more medication prescriptions, and that 20% of these patients are prescribed more than 4 medications23. A cross-sectional analysis of American patients within the HIV Outpatient Study (HOPS) cohort showed that 95% of the patients older than 60 years were polymedicated, which was due to concomitant medication in 73% of these patients24.
A recently introduced analogous concept is pill burden, which includes the number of medications and the number of pills. Zhou et al. were the first authors to discuss pill burden in HIV-positive patients25. The results showed that the median number of medications per participant was 8 (interquartile range (IQR): 6-11). The median individual daily pill burden was 8 pills (IQR: 5-15), which comprised 3 antiretrovirals (ARVs) (IQR: 2-5) and 6 non-ARVs (IQR: 3-12.5). The duration of antiretroviral treatment (ART) (for every 2 years in treatment) and more than 3 comorbidities was significantly associated with high pill burden (more than 10 pills per day). In a similar analysis in a French cohort, Cuzin et al. found that 62% of HIV patients older than 50 years had comorbidities and 71% were receiving comedication26. In a Spanish study, Gimeno-Gracia et al. investigated the use of concomitant medication in HIV- positive patients compared to the general population. In descending order of frequency, the most commonly prescribed families of medications were angiotensin-converting enzyme inhibitors, antidiabetics, statins, antithrombotic agents, and calcium channel blockers6. It has been found that antiretroviral therapy (ART) strategies are also affected by polypharmacy. Guaraldi et al. suggested that single-tablet-regimens strategies are less likely to be prescribed in patients with simultaneous prescriptions for several concomitant drugs because of the risk of drug-drug interactions, among other factors27. The Italian GEPPO cohort study also confirmed that ARV regimens in elderly HIV patients are tailored according to polypharmacy16.
Recently, attempts have been made to pass from the analysis of the qualitative aspects of polypharmacy, particularly in HIV patients, to its quantitative aspects by analysing numeric data that can subsequently be used to measure healthcare outcomes. This aspect was analysed in the Pharmacy Practice Model Summit 2011 document published by the American Society of Hospital Pharmacy (ASHP), which recommended that the pharmacotherapeutic follow-up of patients should be conducted based on a patient medication complexity index (CI). The CI should include factors such as the severity of the disease, number of medications, and comorbidities28. Up to this point in time, Martin et al. (2007) had published the only CI, which was designed to assess ARVs9. This CI provided a score based on the weighting of 15 variables grouped into four main blocks: dosing schedules, administration methods, special instructions, and required preparations. Since then, the University of Colorado has continued to develop the tool to simplify its calculation and extrapolate it to any other pathology. This project led to the development of the Patientlevel Medication Regimen Complexity Index (pMRCI) and the Antiretroviral Medication Regimen Complexity Index
(ARCI)10,11,29, which include scores ranging from 0.75 to infinity depending on the prescribed medication.
In a HIVpositive cohort, Metz et al. found that the pMRCI scores ranged from 2 to 67.5 (ARVs contributed approximately 25% to the total value)11. Several authors have analysed the relationship between CIs and different health outcomes. MonjeAgudo et al. found that a CI score of 5 marked the threshold above which patients had a higher risk of ART discontinuation30. Jiménez-Galán et al. analysed the relationship between medication complexity and adherence with therapeutic objectives in HIV-positive patients with dyslipidaemia. They found that although most patients met the objectives for ART, almost half of them did not meet the therapeutic objectives of dyslipidaemia treatment, especially those with a higher CI31. Calvo-Cidoncha et al. confirmed that the addition of antihepatitis C therapy to ART in HIV-hepatitis C virus (HCV) co-infected patients led to an increase in medication regimen complexity and the risk of blips, and a decrease in adherence to ART32.
Clearly, patient treatment adherence is one of the main aspects to consider regarding the repercussions of medication regimen complexity. Adherence has already been investigated in general population studies, whose results are of interest because of their possible relevance to the HIV-positive population33. A recent systematic review showed that a lack of adherence was associated with polypharmacy12. Although some of these studies suggested that adherence rates tend to be better in older HIV-positive patients than in younger individuals, it has been observed that cognitive deterioration can hinder this objective. In addition, polypharmacy can contribute to regimen fatigue34. The study conducted by Cantudo-Cuenca et al. in the HIV-positive population showed that the concomitant use of medication decreases adherence to ARVs35.
Another factor highlighted in the literature is the risk of drug interactions, which can be seen to increase in older HIV- positive patients due to treatments for multiple comorbidities that appear in this population36-38. Furthermore, it is already known that most ARVs and other commonly used medications share a metabolic pathway at the level of cytochrome P450 and P-glycoprotein39.
Polypharmacy and its impact on interactions have been described in studies such as that by Holtzman et al. in an American cohort of HIV-infected outpatients24. Slightly more than 3800 patients were analysed over a 5-year observation period. Of these patients, 7% were prescribed at least 1 contraindicated ARV/non-ARV combination. The main contraindicated non-ARVs were proton pump inhibitors, statins, and benzodiazepines. Another key finding of this study was that almost 30% of HIV patients older than 50 years were using medicinal herbs, vitamins, and other supplements that can interact with ARVs, as shown in other studies40-44.
In clinical terms, a basic issue has been to identify how the medication combinations used to treat chronic diseases and ART in older HIV-infected adults increases the risk of clinically relevant interactions which can lead to the loss of drug efficacy, virological failure, and toxicity. It has been found that older patients are more susceptible to interactions than younger individuals19. Firstly, older patients have more age-related comorbidities. Secondly, age-related physiological changes affect the pharmacokinetic and pharmacodynamic properties of medications. These physiological changes, which are also seen in HIV-positive patients, can be explained by a set of factors that include the patient’s genetics, lifestyle, and specific environment. Age-related pharmacokinetic changes are due to changes in the body mass index and in the functioning of organs that eliminate medications. Older age is associated with decreased hepatic volume and hepatic blood flow19, as well as creatinine clearance, leading to the decreased elimination of medications and thus their accumulation. The glomerular filtration rate decreases by about 1% per year as age increases. Current methods to estimate renal function may lead to it being overestimated in older adults if low muscle mass is not taken into account45-46. This problem is further complicated in HIV-infected adults because they typically have less muscle mass than non-HIV-positive adults. Another aspect to consider is that renal function can also be decreased by other pathologies or factors that act as confounders, such as diabetes mellitus, hypertension, low CD4 cell count, race, and the use of ARVs47-48. Thus, the estimation of renal function is more difficult in HIV-infected older adults, and affects the dosage and prescription of drugs excreted by the kidneys19.
Frailty is another key concept of increasing relevance in the management of HIV patients. It is an emerging syndrome which has been shown to be a good predictor of worse health status and adverse events in the general population13,14. Frailty embodies a biological syndrome of diminished functional reserves, altered homeostatic capacity, and reduced resistance to external stressors. It is the result of an accumulation of deficiencies in physiological systems and increases vulnerability to various adverse outcomes, including falls, delirium, hospitalization, disability, and death15. It is known that HIV-positive patients experience immune changes similar to those caused by aging in the uninfected elderly population. These changes occur as a result of a baseline state of immune activation and persistent inflammation that gradually lead to the premature aging of the immune system known as immunosenescence48. Chronic activation of the immune system is marked by persistent viral replication in reservoirs, coinfections with other viruses, and particularly by bacterial translocation due to persistent alteration of the intestinal barrier48.
These aspects have led to the new concept of “potentially inappropriate medications” (PIMs)49. Their use involves the risk of patients experiencing adverse events which outweigh the clinical benefits of the PIMs, particularly in settings in which there are safer and more effective therapeutic alternatives. PIMs also include medications that are used more frequently or for a longer duration than indicated, the use of medications with a high risk of drug-drug interactions, the duplication of medications of the same class, the incorrect selection of the drug or dose, and the non-use or underuse of beneficial medications that are clinically indicated.
In the last decade, there has been growing interest in developing criteria to define the appropriateness of pharmacological treatment in the elderly. Different groups of criteria have been developed for the detection of PIMs in elderly patients, such as the Beers criteria and the Screening Tool of Older Person’s Prescriptions/Screening Tool to Alert doctors to Right Treatment (STOPP/START) criteria50. The application of these criteria to improve the use of medicines and avoid the use of PIMs in the HIV-positive population has been investigated and validated in several studies. McNicholl et al. recently identified potentially inadequate prescribing (PIP) in 54% and 63% of HIV-positive patients older than 50 years, using the STOPP and Beers criteria, respectively51. Although the study by Greene et al.52 used a smaller sample than the previous study, it provided the first hints of aspects such as PIMs. PIMs were assessed by applying the Beers criteria, which includes a guide list of medication and classes of medications that should be avoided in patients older than 65 years. Likewise, so-called anticholinergic risk was assessed using the validated anticholinergic risk scale (ARS) scale. This instrument is scored on a scale ranging from 0 to 3, where 3 indicates high risk and 0 indicates no risk. The highest scores predicted a high risk of falls and mental disorders in the elderly population. The study found that 52% of patients were receiving PIMs and 17% had an anticholinergic risk score of 3. In real-world clinical practice, Casajús-Navasal et al. used the anticholinergic burden (ACB) scale and ARS scale to measure anticholinergic risk in a cohort of Spanish patients older than 50 years old. In total, 43.3% and 36.4% of patients had high anticholinergic risk on the ACB and ARS scales, respectively53.
Deprescribing is a concept that is well known in the setting of the HIV-negative elderly population54-57. Scott et al.58 defined it as the systematic process of identifying and discontinuing medications in cases in which current or potential harm outweighs current or potential benefit within the setting of individualized healthcare that takes into account the patient’s current level of functioning, life expectancy, values, and preferences. This process of drug withdrawal has the potential benefit of reducing the harmful effects associated with polypharmacy, while ensuring the ability of the patient to continue receiving the most appropriate therapy59. Studies have identified several barriers to reducing the number of medications and to facilitating deprescribing60,61. Currently, there is a wealth of information on when to start a therapy, including in the HIV-positive patient, but there is very little on how and when to stop it. Despite a lack of evidence in support of therapy being continued, the literature typically describes the continuation of the prescribed treatments60,61.
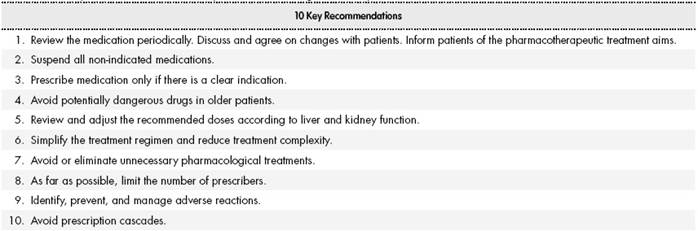
These new approaches to the management of medication derive from the concept of optimization of pharmacotherapy and the inclusion and application of these new concepts. This has led to a consensus on the need to review all prescribed medications at least every 6 months in elderly HIV-positive patients who have been prescribed 4 or more medications, and at least once a year in the remaining patients19. An emerging priority is to identify and establish strategies to reduce medication regimen complexity and avoid polypharmacy in elderly HIV-positive patients to the greatest extent possible. It is recommended that the review of prescribed medications should be conducted in a systematic manner using a sequential and structured methodology. Table 2 and Table 3 show recommendations extracted from the literature on optimizing prescription strategies and introducing a review of polypharmacy in daily clinical practice.
Discussion
The management of HIV-positive patients, especially elderly ones, is of growing concern, as shown by the increasing number of articles addressing this problem in recent years. However, we need to deepen our understanding of the new concepts and to confirm the results based on them, given that the definitions used, particularly those of polypharmacy and medication regimen complexity, and the comparative analysis methodology applied have not yet been standardised. Additionally, these new concepts, which have been identified as challenges by the professionals that serve this population, require a refocusing on care management in all professional settings. A priority issue is to avoid an exclusive focus on the treatment of the infection and the management of ARVs (adherence, interactions, and adverse effects). The literature analysed shows an increasing number of emerging problems associated with aging, such as the management of comorbidities and functional and cognitive impairment. Furthermore, the physiological changes associated with aging must be taken into account in order to optimize pharmacotherapy and the clinical monitoring of these types of patients. According to the literature, such changes are not linear or consistent. Therefore, a global approach is needed that can identify elderly HIV-positive individuals, who are at risk of aging with a worse state of health or, equivalently, who are at risk of having a worse quality of life. The first available data on the application of these concepts in the older non-HIV population confirm a high rate of PIPs and elevated severe anticholinergic risk.
No study has addressed deprescribing in this population, but given the results published by various authors, a strategy is needed to implement this strategy and to measure its usefulness.
The main limitations found in the analysis were the use of different populations, age thresholds, and study methodologies. Similarly, the different definitions used and their lack of standardization make it difficult to compare different studies that have addressed similar concepts, especially polypharmacy and medication regimen complexity.
Future studies will confirm how the approach proposed by some authors will lead to a new vision of these patients and their management. Evidence is already urgently needed that would demonstrate that this new way of understanding the pathology leads to improvements in the health setting and in the patients themselves, given that such evidence is not currently available. In conclusion, there is a growing interest in deepening our understanding of the relationship between HIV infection and aging. Medication regimen complexity is beginning to be used as a criterion for pharmacotherapeutic follow-up because of its influence on health outcomes. New concepts have to be incorporated and applied that will improve pharmacotherapeutic optimization in this population.











 texto en
texto en