Introduction
The treatment of infected and colonized wounds requires a multidisciplinary approach that includes, among other types of care, surgical debridement to remove necrotic and devitalized tissue, suitable systemic antibiotic treatment, and wound cleaning to eliminate the exudate, reduce the bacterial load, and encourage the formation of granulation tissue on the wound bed to close or cover it safely, depending on the complexity of the wound1.
Negative pressure wound therapy (NPWT) is of assistance in the treatment of wounds, because it promotes granulation of the wound bed, eliminates exudate, and prepares the wound for closure. The topical administration of solutions in combination with NPWT -known as negative pressure wound therapy with instillation (NPWTi)- further aids wound cleaning and microbial eradication.
The first known use of NPWT dates from 1550 BC in Egypt, where cupping therapy2 was used to apply suction to the surface of wounds. There are also records of its use in China (1000 BC), and Hippocrates (400 BC) applied it to structural problems and skin diseases2.
In 1998, NPTW with antiseptic and antimicrobial solutions (NPTWi) was introduced to treat infected wounds unresponsive to conventional therapy3.
Currently, a range of NPWT and NPWTi devices are available. The NPWTi devices provide automated volumetric delivery of antiseptic or antimicrobial solutions. In this approach, instillation is alternated with negative pressure. NPWTi systems provide intermittent administration of a predefined amount of solution that remains on the wound for an amount of time set by the user before negative pressure is applied again. The level of accuracy provided by the devices has positioned NPWTi as a first-line therapy in the treatment of complex wounds.
The use of NPWT and NPWTi has proven benefits, which include the promotion of angiogenesis and granulation tissue formation, the stimulation of cellular and local blood flow, and the reduction of exudate and oedema. In addition, pain is reduced due to the elimination of lactic acid and the reduction of infectious burden. The wound is also enclosed in a moist and closed environment4.
Over the last 15 years, these therapies have improved the care of patients with chronic and acute wounds, and are currently a relevant tool for health care professionals. These therapies cover a wide range of clinical applications, of which the most relevant are prosthesis infection, surgical wounds or amputations, and pressure and diabetic foot ulcers.
However, there is limited evidence in the literature in support of their effectiveness, safety, and efficiency. Authors and health professionals support their use given their positive outcomes and cost reductions. Their support is based on reviews and experience in which NPWTi with antiseptics and NPWT were used; however, data on instillation with antibiotics are still very scarce. Likewise, there are few guidelines or recommendations based on consensual protocols that could standardise their use.
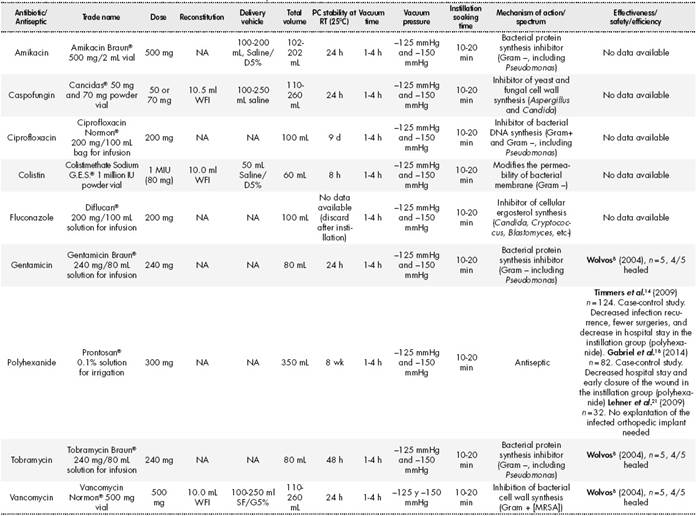
According to reported experience, the antiseptic solutions most commonly used were polyhexanide (0.005%-0.040% solution), acetic acid (0.25%-1.00% solution), and povidone-iodine (10% solution)5. Regarding antibiotic instillation, several authors have reported the use of bacitracin/ neomycin6, polymyxin B/bacitracin7, vancomycin, gentamicin, and tobramycin8. In the United States, their use in NPWTi therapy is considered to be off-label9. The antimicrobial action of the instilled antibiotics or antiseptics is assumed to be local on the wound bed, with no evidence of systemic absorption.
This study was conducted in the setting of a Spanish Mutual Society Hospital that provides care to patients with workplace injuries. In 2017, the hospital performed 1,522 interventions in the Plastic and Reconstructive Surgery department and 4,130 interventions in the Orthopaedic and Traumatology Surgery department. In total, 6 patients with post-surgical wounds received NPWT and 7 received NPWTi. Four patients received antibiotic instillation therapy with colistin, gentamicin, and vancomycin after the isolation of microorganisms such as Acinetobacter baumannii, methicillin-resistant Staphylococcus aureus, Staphylococcus epidermidis, Proteus mirabilis, and Enterobacter cloacae. The following aspects were not defined: vehicle, stability, duration of solution on the wound (soaking time (ST)), negative pressure values (vacuum pressure (VP)), and duration of negative pressure delivery (vacuum time (VT)).
The aim of this study was to establish recommendations for the use of NPWTi therapy in our hospital based on evidence on effectiveness, safety, and efficiency, the consensus guidelines available in the literature, and the verified stability of all the instillation solutions.
Methods
Effectiveness, safety, and efficiency of NPWTi
We conducted a literature search for clinical studies published in the last 30 years that had assessed the effectiveness, safety, and efficiency of NPWTi therapy. The PubMed and Cochrane databases were searched using the keywords “drug instillation”, “wound”, “infection”, “negative pressure wound therapy”, “closure”, “vacuum”, and “anti-infective agents”. The search was not limited by language, year of publication, or type of publication.
Consensus guidelines on NPWTi
We conducted a bibliographic search for any international consensus guidelines with specific recommendations on NPWTi therapies that had been published in the last 30 years. The PubMed and Cochrane databases were searched using the keywords “instillation drug”, “wound”, “infection”, “negative pressure wound therapy”, “closure”, “vacuum”, “anti-infective agents”, “international”, “consensus”, and “guidelines”. This search was not limited by language, year of publication, or type of publication. We only selected guidelines that were agreed by multi-disciplinary consensus based on the review of the available literature and the clinical experience of the team members themselves.
Level of evidence
The level of evidence of studies and reviews on effectiveness, safety, and efficiency of NPWTi therapies was established using the Evidence Rating Scale for Therapeutic Studies of the American Society of Plastic and Reconstructive Surgery (ASPS)10, which classifies the level of evidence into 5 categories (I to V) according to the study design (Table 1).
Table 1. Classification Scale of Levels of Evidence for Therapeutic Studies According to the American Society of Plastic and Reconstructive Surgery10

Use recommendations for NPWTi
Based on the available literature on effectiveness, safety, and efficiency, the consensus guidelines, and the verified physicochemical stability of all the instillation solutions, we drafted a set of use recommendations for NPWTi in all patients beginning local treatment of the wound. Each antiseptic or antibiotic used for instillation was classified according to brand name, mechanism and range of action, dose and dosage, reconstitution conditions, vehicle, total preparation volume, physical and chemical stability at room temperature, VP, VT, ST, and the available evidence on effectiveness, safety, and efficiency. We only included the antiseptics and antibiotics available for instillation in NPWTi treatment listed in the pharmacotherapeutic guide of the hospital. Recommendations related to the stability of the instillation solutions were based on the Summary of Product Characteristics, the Stabilis database11, USP Chapter 79712, and information on the stability of parenteral preparations13.
Results
Effectiveness, safety, and efficiency of NPWTi
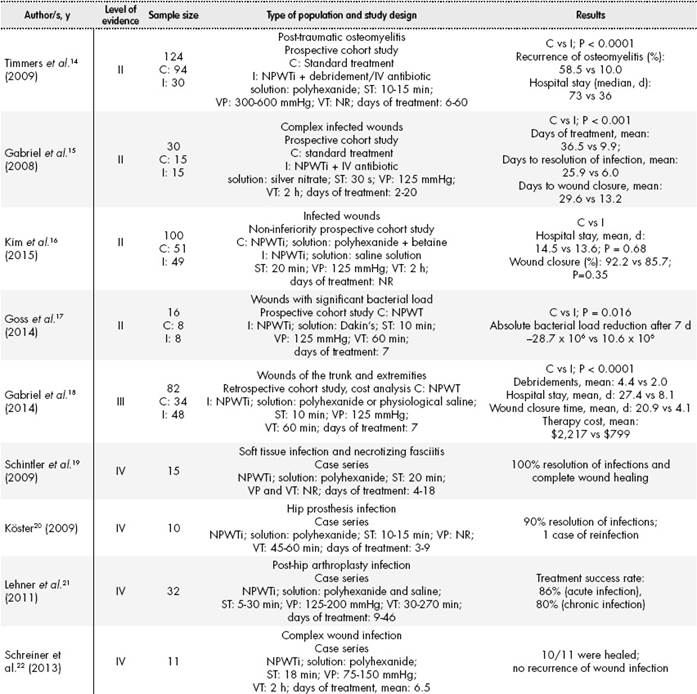
After the bibliographic review of clinical trials associated with the use of NPWTi, 13 articles were selected; these appear in Table 2-1 and Table 2-2 ordered by their level of evidence. Those articles dealing only with NPWT were excluded, as well as those about NPWTi where the type of instillation solution was not mentioned, or where saline was used exclusively.
Five of the studies referenced (Table 2-1 and Table 2-2) were comparative cohort studies (Level of Evidence II and III)14-18, and the rest were from series of cases (Level of Evidence IV)3,6-8,19-22.
In the cohort studies, NPWTi therapy (solutions: silver nitrate or polyhexanide) was compared with standard treatment (debridement, moist wound healing, oral and/or intravenous antibiotic therapy) or NPWT. In terms of efficacy and safety, comparative studies showed statistical significance towards instillation therapies (NPWTi), regarding clinical variables such as infection recurrence14, length of hospital stay14,16,18, time for wound healing15,18 or the absolute reduction in bacterial load in the infection bed17. Specifically, the study by Gabriel et al.18 (n=82) appears interesting; besides efficacy and safety, there was also an evaluation of NPWTi therapy (with polyhexanide or saline) vs. NPWT, and the conclusion was favorable to therapy with instillation.
In the rest of non-comparative studies, we found cases of antiseptic solution instillation (polyhexanide)19-22 and other studies where antibiotic solutions were applied, specifically neomycin / bacitracin6 (n=5), polymyxin B/bacitracin7 (n=5) or vancomycin8 (n=5). As shown in Table 2-1 and Table 2-2, VP, VT and RT were different for each type of study, showing that there is no consensus or standard practice for this therapy.
Table 2. (cont.). Effectiveness, Safety, and Efficiency of NPTWi
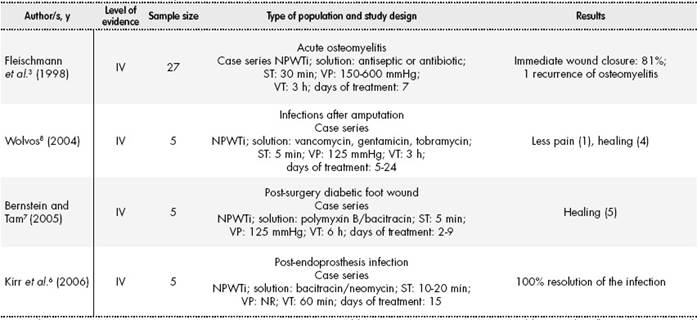
C: control group; I: intervention group; IV: intravenous; NPWT: negative pressure wound therapy; NPWTi: negative pressure wound therapy with instillation; NR: not reported; ST: soaking time of antiseptic/antibiotic; VP: vacuum pressure; VT: vacuum time.
Consensus guidelines on NPWTi
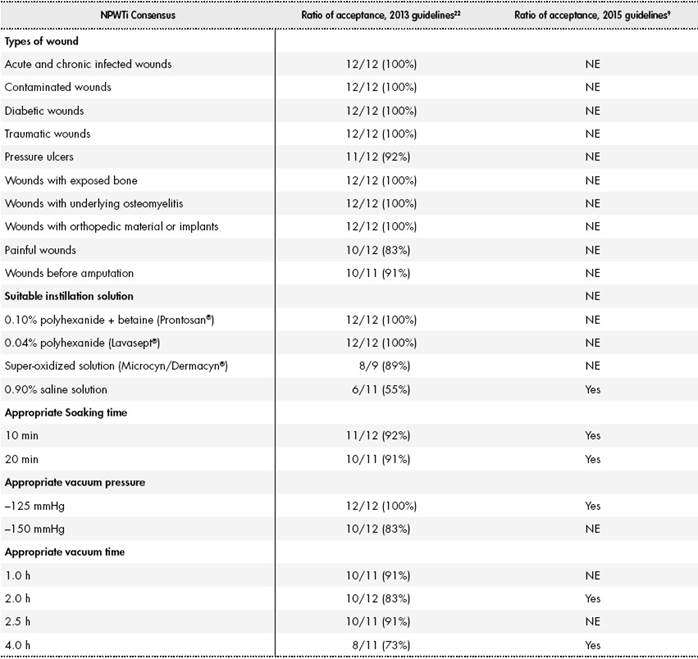
After the literature review, we found 2 expert consensus recommendations on NPWTi9,23. In both cases, multidisciplinary teams comprising general, orthopedic, vascular, and plastic surgeons from the United States and Europe were asked to provide consensus guidelines on the appropriate use of negative pressure therapy with instillation. Several face-to-face meetings were held in which the team discussed the available evidence and individual clinical experience. The final recommendations on the use of NPWTi therapy were the result of agreements reached by over 80% of the members. The 2013 recommendations22 stated that 12 professionals took part in the meetings, whereas the 2015 recommendations provided no information on this aspect9. No guidelines were provided on the instillation of antibiotics or on the stability of antibiotic or antiseptic solutions. Based on the opinions of both teams, we created the following use recommendations (Table 3).
According to the 2013 consensus22, NPWTi would be appropriate for different types of wounds (Table 3). Regarding instillation solutions, the 2013 recommendations supported the use of polyhexanide and hydrogen peroxide, whereas the 2015 recommendations supported the instillation of physiological saline solution. The 2013 recommendations established a VP of -125 mmHg to -150 mmHg, whereas the 2015 recommendations suggested a VP of -125 mmHg alone. The recommended VT varied between 1 hour and 4 hours, but consensus was achieved on an ST of 10 minutes to 20 minutes (see Table 3).
It should be noted that both meetings were sponsored by an instillation device manufacturer, who also selected the team members. Thus, there could be potential conflicts of interest in the 2 studies.
Use recommendations for NPWTi
Based on the available evidence on effectiveness, safety, and efficiency and on the consensus guidelines, we proposed and established some use recommendations to be implemented in our hospital (Table 4). After initiating NPTWi therapy, microbiological samples should be taken from the wound bed every 72 hours to 96 hours. Whenever possible, cultures should be taken during debridement or wound cleaning in the operating room. The duration of treatment with NPWTi therapy should be guided by the clinical judgement of the health professional depending on the status of wound closure, confirmation of negative microbiological growth in 2 or more consecutive samples, or the wound being prepared for a grafting procedure22.
Discussion
The review shows that there is limited evidence in the literature in support of NPTWi. We established some use recommendations based on the classification of the level of evidence of the references and experts opinions mentioned above.
The updated data on antimicrobial solution stability provide added value to patient care by increasing the safety of the process without compromising the effectiveness of the therapy.
The authors are fully aware of the limitations of this study. Firstly, these recommendations are limited to the specific situation of our hospital; thus, it may not be possible to extrapolate them to other settings or professional users. Secondly, this article does not report the results of implementing these recommendations, since the study was restricted to issuing guidelines based on the literature review and expert opinions. Finally, it should also be mentioned that some publications, especially the reviews and consensus opinions, were sponsored and funded by the American manufacturer of the devices, which may involve potential conflicts of interest in their recommendations.
Therefore, further research is needed to determine which instillation solutions are the most suitable in given clinical settings, particularly in the field of antimicrobials, for which there is very limited information.
Although this article establishes a set of updated recommendations on VP, VT, and ST, new studies are required to confirm whether these recommendations are generalizable, or if, on the contrary, exceptions should be made according to the clinical situation
Contribution to the scientific literature
This article provides preliminary guidelines on the application of negative pressure therapy with instillation based on evidence regarding its effectiveness, safety, and efficiency and according to the consensus guidelines available in the literature. These recommendations were also based on a review of the physicochemical stability of the antiseptic and antimicrobial solutions most commonly used for instillation. This information is typically requested by prescribing physicians.
The contribution of this review is to increase awareness of the recommendations for negative pressure therapy with instillation among health professionals in their clinical practice. The correct and rational use of available resources would lead to better health outcomes.











 text in
text in