Introduction
In 2012, a new measure on the additional monitoring of specific medicines was introduced in the European legislation on pharmacovigilance1,2. This measure affects medicines authorized by the European Medicines Agency (EMA) in the European Union that require particularly rigorous and intensive monitoring by the health authorities.
Medicines Under Additional Monitoring (MUAM) are identified with an inverted black triangle ( ). Additional monitoring status is applied to a medicine in the following cases3: 1) it contains a new active substance that was authorized in the European Union after January 1, 2011; 2) it is a biological medicine that was authorized in the European Union after January 1, 2011; 3) it has been given conditional authorization, for which the Marketing Authorisation Holder (MAH) must provide additional data; 4) it has been given conditional authorisation under exceptional circumstances (when there are specific reasons why the MAH cannot provide a comprehensive dataset and must complete the information after authorisation); and 5) it is a medicine for which the MAH must conduct post-authorization safety studies (PASS). Currently, MUAMs remain subject to additional monitoring for five years after their authorisation or until the EMA Pharmacovigilance Risk Assessment Committee (PRAC) considers that it is safe to withdraw them from the list of MUAMs.
). Additional monitoring status is applied to a medicine in the following cases3: 1) it contains a new active substance that was authorized in the European Union after January 1, 2011; 2) it is a biological medicine that was authorized in the European Union after January 1, 2011; 3) it has been given conditional authorization, for which the Marketing Authorisation Holder (MAH) must provide additional data; 4) it has been given conditional authorisation under exceptional circumstances (when there are specific reasons why the MAH cannot provide a comprehensive dataset and must complete the information after authorisation); and 5) it is a medicine for which the MAH must conduct post-authorization safety studies (PASS). Currently, MUAMs remain subject to additional monitoring for five years after their authorisation or until the EMA Pharmacovigilance Risk Assessment Committee (PRAC) considers that it is safe to withdraw them from the list of MUAMs.
In April 2013, the EMA published the first MUAM list, which is reviewed monthly by the PRAC3. In 2013, the Spanish Agency of Medicines and Medical Devices (AEMPS in Spanish) published two information notes on this measure that were aimed at healthcare professionals4 and citizens5, respectively. In addition, some therapeutic and pharmacovigilance bulletins have reported on the MUAM concept and procedure6-8, and some published articles have addressed safety and other aspects related to the use of specific MUAMs9-13.
The overall objective of this study was to analyse the characteristics of MUAMs. Specifically, we assessed the following aspects: the criteria applied to approve a medicine as a MUAM; the dispensing criteria assigned to these medicines; the pharmacological groups to which they belong; and their post-authorisation safety.
Methods
We conducted a descriptive analysis of the EMA MUAM list, which was updated on January 23, 201714. Medicines whose authorization had been revoked or suspended were excluded. Medicines authorized between January 23, 2012 and January 23, 2017 were considered to be new active substances. The following websites were used as information sources: 1) the EMA14 (list of MUAMs, authorization dates and Summary of Product Characteristics, and information related to the authorization criteria); 2) the AEMPS Medicine Information Center15 (conditions for dispensing medicines); and 3) the Norwegian Institute of Public Health, WHO Collaborating Centre for Drug Statistics Methodology16 (Anatomical-Therapeutic-Chemical (ATC) classification code corresponding to each active principle).
Each MUAM was analysed according to whether: 1) it was a new active substance; 2) it was a biological drug, biosimilar or otherwise, excluding those already included as new active substances; and 3) other criteria had been applied to approve the medicine as a MUAM (conditional authorization, authorization in exceptional circumstances, PASS). We also analysed the following aspects: 1) the temporal evolution (2013-2016) of the MUAM assignment criteria; 2) assignment as an “orphan drug” or otherwise; 3) the authorized dispensing conditions, classified as “unrestricted” (MSMP: medicines subject to medical prescription) and “restricted” (DH: hospital diagnosis and H: hospital use); and 4) the pharmacological group to which they belong: 1st level (organ and system) and 2nd level (therapeutic subgroup) of the ATC classification corresponding to each active principle.
For the purposes of the PASS, we reviewed the information notes on MUAM safety published by the AEMPS until January 23, 2017, and analysed the reasons for issuing the information note. This information was classified according to its content: 1) RBR: results of the risk/benefit analysis of the medicine; 2) CDC: changes in the dispensing criteria; 3) NCI: new contraindications; 4) RU: restrictions on use; 5) SAI: safety information.
Data on each variable defined in the study were cleaned and statistical analyses were conducted using the IBM SPSS Statistics software package version 24.0 (IBM Corp, Armonk, New York). The majority of the results are expressed as absolute (n) and relative frequencies (%).
Results
The MUAM list (EMA/245297/2013 Rev.41) was updated on January 23, 2017 and included 320 medicines. Of these, 4 were excluded because their marketing authorization was revoked or suspended after they had been included in the list. Of the 316 remaining MUAMs, 197 (62.3%) contained new active substances (Table 1). Other inclusion criteria were as follows: the need for a PASS (n = 52 (16.5%)); and being a biological medicine, but not a new active ingredient (n = 49 (15.5%)). Of the total of 197 MUAMs containing new active substances, at least 1 additional criterion was applied to 55 (24.9%) of them. Overall, almost 66% of the MUAMs (n = 207 (65.3%)) were authorized under restricted dispensing conditions, with hospital use being the most common dispensing criterion (n = 143 (45.1%)).
Table 1. Characteristics of the medicines under additional monitoring (List EMA/245297/2013 Rev.41)
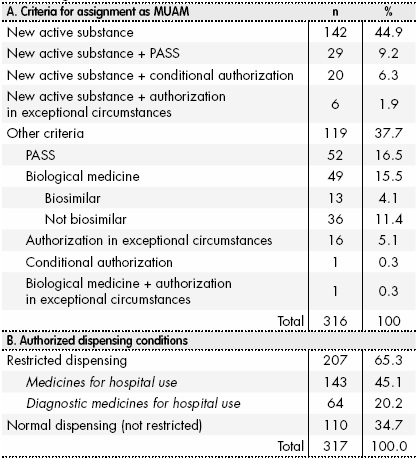
MUAM: medicines under additional monitoring; PASS: post-authorisation safety study.
Of the 197 MUAMs containing new active substances, 53 (26.9%) were new biological active substances and 47 (23.9%) were orphan medicines (18 biological orphan medicines and 29 non-biological orphan medicines). The new active substances of the MUAMs analysed mainly belonged to the 1st level of the ATC classification (anatomical main group) (Figure 1): antineoplastic and immunomodulatory agents (L) (n = 59 (29.9%)), antiinfective agents for systemic use (J) (n = 32 (16.2%)); and drugs for metabolism and the alimentary tract (A) (n = 30 (15.2%)). Within the group of antineoplastic and immunomodulatory agents, the most common pharmacological group were tyrosine kinase inhibitors (n = 24 (12.2%)).

Figure 1 Anatomical-therapeutics-chemistry (ATC) classification of medicines under additional monitoring with new active substances in their composition.
The analysis of the temporal evolution of the criteria used to assign medicines as MUAMs over the period 2013 to 2016 showed the following: 1) the criterion “new active substance” applied to at least half of the medicines assigned as MUAMs each year during the study period; 2) the criterion “biological medicine” but not a new active substance applied to 32.3% (its highest value) of the medicines assigned as MUAMs in 2016; 3) the criteria “conditional authorization” and “authorization in exceptional circumstances” applied to less than 3% of MUAMs in the period 2014 to 2016; and 4) there was a decrease in the number of medicines fulfilling the “PASS” criterion in 2015 and 2016 (only 3.2% of the MUAMs in 2016) (Table 2).
Table 2 Temporal evolution of the criteria used to assign medicines under additional monitoring

AEC: authorization in exceptional circumstances; CA: conditional authorization; PASS: post-authorization safety study.
From the time of publication of the first MUAM safety information note on June 3, 2013 (MUH (FV), 13/2013) to January 23, 2017, the AEMPS published 68 safety information notes, of which 2 explained the concept of MUAMs to healthcare professionals and citizens and 14 (20.6%) referred to some aspect related to the safety of the MUAMs involved (Table 3). These 14 informative notes referred to the following aspects: new information on safety (7), restrictions on the use of the drug (3), the results of the risk/ benefit analysis (2), changes in the medicine dispensing conditions (1), and new contraindications (1). Three of the informative notes referred to strontium ranelate (change in its dispensing conditions to a diagnostic drug for hospital use) and 2 referred to idelalisib (measures to prevent the risk of severe infection). These 2 active substances were the ones more often referred to in the notes.
Table 3 Safety information notes on medicines under additional monitoring issued by the Spanish Agency of Medicines and Medical Devices until January 23, 2017

BBR: risk/benefit ratio; CD: dispensing criteria; CNT: contraindications; FV: pharmacovigilance; MUH: medicines for human use; RU: restricted use; SEG: safety. * Year of authorization.
Discussion
The inverted black triangle symbol ( ) used in the United Kingdom to identify new active substances17 was adopted by the EMA to identify MUAMs. After the adoption of the European regulation, the AEMPS eliminated the yellow triangle (
) used in the United Kingdom to identify new active substances17 was adopted by the EMA to identify MUAMs. After the adoption of the European regulation, the AEMPS eliminated the yellow triangle ( ) that had been used in Spain to indicate new active substances. Assigning the inverted black triangle (
) that had been used in Spain to indicate new active substances. Assigning the inverted black triangle ( ) to a medicine does not mean that it is unsafe. Its purpose is to encourage health professionals18 and citizens19 to report suspected adverse reactions associated with the medicine, thus facilitating the benefit-risk analysis.
) to a medicine does not mean that it is unsafe. Its purpose is to encourage health professionals18 and citizens19 to report suspected adverse reactions associated with the medicine, thus facilitating the benefit-risk analysis.
The present study found that almost 66% of MUAMs contained new active substances. Thus, there is a need to complete the information on their safety20. The percentage of MUAMs with new active substances remained equal to or greater than 50% during each year of the study period. These new active substances mainly belonged to the group of antineoplastic and immunomodulatory medicines. Among these medicines, tyrosine kinase inhibitors deserve special mention due to the frequency of new active substances21,22.
One-third of the MUAMs were biological medicines, equally divided into new active substances and 13 biosimilars. Biological medicines include proteins (e.g. peptide hormones), enzymes produced naturally by the human body, monoclonal antibodies, and blood products. They also include immunological medicines (e.g. serums, vaccines, and allergens), and advanced technological products (e.g. gene and cell therapy products)23-25. Due to their chemical characteristics and safety profiles26,27, pharmacovigilance plays a critical role in the setting of biological medicines28.
A high percentage of MUAMs were subject to restricted dispensing (mainly for hospital use) and almost all orphan medicines were under restricted dispensing. Orphan medicines are used to treat rare diseases that affect only a small number of patients (< 5/10 000 individuals in the EU or < 200 000 individuals in the USA)29. Approximately 20% of MUAMs were authorized as orphan medicines, of which more than half were new active substances that mainly comprised antineoplastic and immunomodulatory agents.
Prior to 2017, the AEMPS had published 14 safety information notes on medicines identified with the inverted black triangle ( )30. All of these notes highlighted the relevance of the additional monitoring of these medicines and the priority notification of suspected adverse reactions.
)30. All of these notes highlighted the relevance of the additional monitoring of these medicines and the priority notification of suspected adverse reactions.
The main limitation of this study is related to dynamic changes to the EMA MUAM list, which is updated every month. This limitation was compensated for by the high number of MUAMs studied, given that all MUAMs authorized from the beginning of this procedure in 2013 to January 2017 were analysed. The main strength of this study is its usefulness to became known MUAM among health professionals. We propose that the MUAM inverted black triangle ( ) should be incorporated in prescription and electronic dispensing systems to facilitate its identification by health care staff.
) should be incorporated in prescription and electronic dispensing systems to facilitate its identification by health care staff.
In conclusion, the MUAMs mainly included new active substances, which, for reasons of safety or conditions of authorization, require close and careful post-authorisation monitoring. The majority of the new substances belong to the antineoplastic and immunomodulatory group of medicines. The updated PASS has already provided new information that has been published in the informative notes of the AEMPS. Knowledge of these medicines and their identification symbol ( ) should facilitate early risk/benefit analyses.
) should facilitate early risk/benefit analyses.
Contribution to the scientific literature
Common criteria used to designate a medicine as being under additional monitoring are that it contains a new active substance, that it requires post-authorisation safety studies or that it is a biological medicinal product. These medicines are commonly used in cancer and immune processes, and their dispensing criteria are often restricted. Post - authorisation monitoring has already generated additional information to ensure their safer use.











 texto en
texto en 


