Introduction
Colorectal cancer (CRC) is the most frequent neoplasia in the digestive system, and its control is currently one of the priorities within public health, given the mortality and morbidity caused. There has been a great evolution in pharmacological treatment during recent years, and 5-fluorouracil (5-FU) has become the basic treatment for patients with non-metastatic CRC (nmCRC). 5-FU has been used in combination with other agents in order to increase survival, specifically with folinic acid or leucovorin (LV)1 , and subsequently with oxaliplatin2.
Another drug used for these patients is capecitabine, a precursor of 5- FU3. Several studies have demonstrated that the use of capecitabine in patients with nmCRC is an alternative option as effective and well tolerated as 5-FU/LV4; therefore, intravenous 5-FU can be replaced by oral capecitabine. The combination of capecitabine and oxaliplatin (XELOX regimen) has also demonstrated an improvement in survival, and has been compared with 5-FU/LV5. When comparing the FOLFOX and XELOX regimens, the study by Schmoll HJ et al. demonstrated that adjuvant treatment with 5-FU/LV or capecitabine with or without oxaliplatin provided optimal results; the conclusion was that both regimens presented equivalent efficacy6.
However, it is also important to analyze the quality of life (QoL) of patients in order to understand the experience of the patient with their disease and its treatment, and to be able to choose a chemotherapy regimen over another7. Therefore, the objective of the study is to evaluate and compare the QoL of patients diagnosed with nmCRC treated with the FOLFOX or XELOX regimen, based on the EORTC QLQ-C30 questionnaire, version 3.0.
Methods
A descriptive prospective study on patients diagnosed with nmCRC receiving adjuvant chemotherapy treatment with the FOLFOX and XELOX regimens. The FOLFOX adjuvant regimen (intravenous oxaliplatin + intravenous 5-FU/LV) consists of 12 14-day cycles during 24 weeks, and the XELOX adjuvant regimen (intravenous oxaliplatin + oral capecitabine) consists of 8 21-day cycles during 24 weeks8. A lower number of cycles could be administered to patients with rectal cancer, if they had received chemoradiotherapy before surgery8.
The study was conducted in a second level hospital during 24 months (October, 2015 to October, 2017), after being authorized by the Ethics Committee for Clinical Research. All patients who initiated and completed the adjuvant chemotherapy treatment and signed the informed consent for participation were included in the study. The patients excluded were those with cognitive impairment that prevented them for understanding and answering questionnaires, patients unable to understand Spanish, and those who did not agree to participate.
Patients were selected at the time of pharmacy validation of their chemotherapy treatments; after signing the Informed Consent, they were given the EORTC QLQ-C30 questionnaire, version 3.0, at that time and at week 12 of initiating adjuvant treatment. This is a questionnaire validated and developed by the European Organisation for Research and Treatment of Cancer Quality of Life, in order to measure the QoL of oncology patients; it consists of 30 questions split into three scales: functional, symptomatic, and overall health status9,10. Afterwards, there was a review of the computerized clinical record (Mambrino XXI®) and pharmacotherapeutic records (Farmatools-Dominion® and Farhos-Oncología® v.5.0).
Exposure variables were collected (chemotherapy regimen administered), as well as control variables (age, gender, location and stage of the disease, ECOG, existence or not of previous chemoradiation, number of cycles received, months since diagnosis until initiation of adjuvant treatment, initiation dose, dose reductions and reasons, and treatment interruptions and reasons), and response variables (questionnaire scores). Regarding the questionnaires, scores were standardized: high values in the functional scale and the overall health status scale pointed at a better QoL, and high values in the symptom scales pointed at a worse QoL11. Any changes in the items and/or scales superior by 10 points to the basal scores were considered clinically relevant. Alterations from 5 to 10 points entailed a “small” change, alterations from 10 to 20 points reported “moderate” changes, and a difference > 20 points involved “high” change12; therefore, only “moderate” and “high” changes involved clinical relevance.
Statistical analysis of data was conducted with the SPSS® 15.0 program (version for Windows®). A descriptive analysis of continuous or numerical variables was conducted by using central tendency and dispersion measures, while absolute and relative frequencies were used for categorical or qualitative variables.
Regarding QoL assessment, the mean value of each of the questionnaire items was obtained from the mean of the questions included. The comparison between mean values in a quantitative variable by another dichotomous qualitative was conducted with the T test for independent samples. A p < 0.05 value was considered statistically significant.
Results
Thirty-six (36) patients were selected; one of them was excluded from the study because he was unable to understand Spanish. Of those 35 patients initially included in the study, five withdrew from the study once adjuvant treatment was initiated: one patient interrupted treatment after the first cycle due to cardiotoxicity, two patients due to disease progression, and another two patients because they did not complete the adjuvant treatment with the same chemotherapy regimen, alternating the FOLFOX and XELOX regimens.
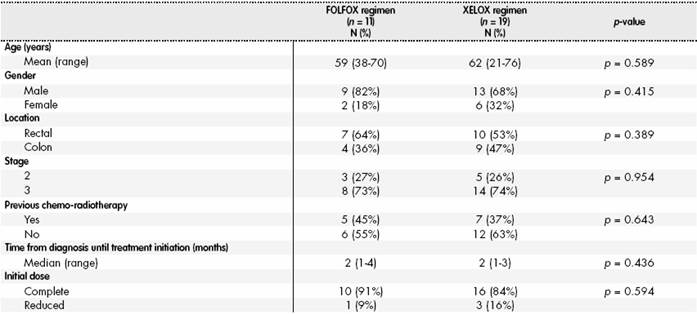
Therefore, 30 patients were included in the analysis; their characteristics, according to the chemotherapy regimen received, appear in Table 1, and no statistically significant differences were found between the different variables.
Regarding dose reductions or interruptions at the end of adjuvant treatment: 22 (73%) patients had reduced their dose and five (17%) patients had interrupted treatment. The main reason was the development of adverse reactions, mostly neurotoxicity (11 patients; 37%), thrombopenia (9 patients; 30%), neutropenia (9 patients; 30%) and mucositis (3 patients; 10%).
The results of the analysis of the different items in the EORTC QLQ - C30 QoL questionnaire appear in Table 2; no statistically significant differences were found in the majority of items. There were statistically significant differences in the emotional role item at 12 weeks of treatment; at this point, patients treated with FOLFOX were better emotionally than those treated with XELOX (FOLFOX 92 points vs. XELOX 82 points; p=0.036).
Table 2. Analysis of the different items in the EORTC QLQ-C30 quality of life questionnaire
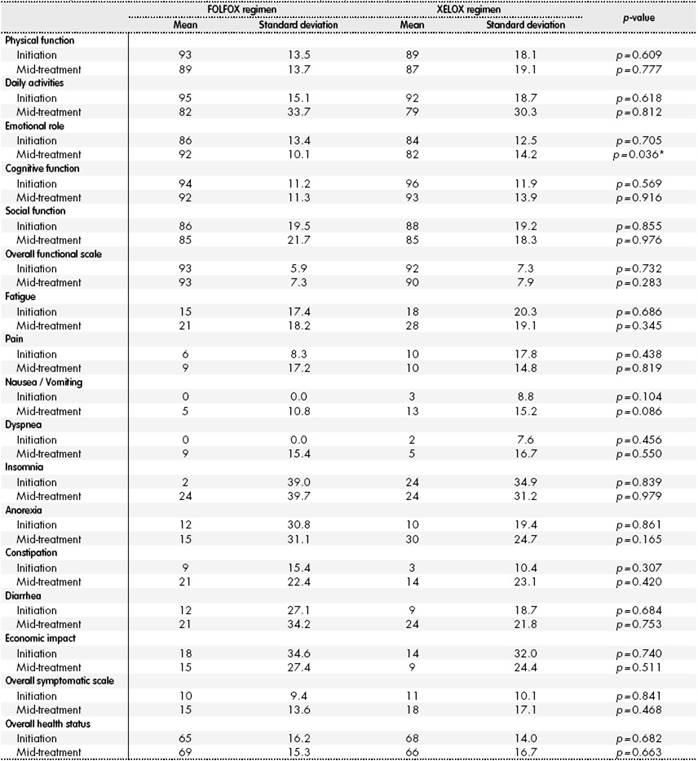
*p < 0.05.
When analyzing the changes perceived by patients throughout their treatment, there were clinically relevant differences regarding the basal value in both patient arms. Patients on FOLFOX presented a clinically relevant worsening in daily activities, constipation and insomnia. Worsening was considered “moderate” for the first two items, and “high” for the last one. There was also a worsening classified as “small”, and therefore not clinically relevant, for the following items: fatigue, nausea, vomiting, dyspnea and diarrhea. When analyzing patients on XELOX, a clinically relevant worsening was observed, and “moderate” in daily activities, constipation, fatigue, nausea, vomiting, anorexia and diarrhea. There was also a worsening considered “small” in economic impact; and therefore, not clinically relevant.
No differences were found in any patient group for these items: physical function, cognitive function, social function, and pain. Regarding the functional scale, symptomatic scale, and overall health scale, there was worsening in the second for both groups, but it was not clinically relevant in any, the change was considered “small”; there was no variation in the other two, with no differences found in any of the patient groups throughout adjuvant treatment.
Discussion
In this study, it was observed that patients treated with XELOX were worse emotionally at week 12 of treatment than those treated with FOLFOX, and presented worsening in fatigue, nausea, vomiting, anorexia and diarrhea.
In comparison with other studies already published, these have demonstrated that patients on FOLFOX presented higher incidence of insomnia and dyspnea vs. XELOX4,13. The study by Comella P et al. in patients with metastatic CRC (mCRC) showed an improvement in insomnia throughout treatment in patients on FOLFOX, and worsening in dyspnea with XELOX14. Regarding the studies by Lin JK et al. and Comella P et al., it should be highlighted that patients on FOLFOX presented an improvement in social function in the first one, and improvement on cognitive function and pain in the second one; while in the second study, there was an improvement in the constipation item for mCRC patients on XELOX4,14.
Regarding anorexia, the study by Chen HH et al. found differences between both regimens, because patients on FOLFOX presented a higher incidence of anorexia both at treatment initiation and at 12 weeks15. In our study, it was observed that there was no worsening in said item throughout adjuvancy with FOLFOX, while there was worsening with XELOX. This difference can be due to the patient profile in each study, because the study by Chen HH et al. analyzed patients diagnosed with mCRC, who presented a higher basal incidence of anorexia than nmCRC patients15.
Finally, the main limitation of the study was its sample size; this fact limits the ability to draw statistically significant conclusions. A larger randomized study could be conducted in order to confirm these results.
Contribution to the scientific literature
Colorectal cancer is the most frequent neoplasia in the digestive system, and its control is currently one of the priorities within public health, given the mortality and morbidity caused. Quality of Life is becoming increasingly considered in Oncology, and it is important to analyze it in order to have a better understanding of the impact of chemotherapy treatment on patient results and, therefore, selecting one chemotherapy regimen over another. To this aim, it is innovative to compare the quality of life associated with the FOLFOX and XELOX regimens in patients with non-metastatic colorectal carcinoma. The quality of life in patients treated with XELOX seems to be worse than that of patients treated with FOLFOX. The highest worsening presented throughout their treatment in the emotional situation of patients on XELOX was caused by a higher perception of fatigue, nausea, vomiting, anorexia and diarrhea. Therefore, at the time of selecting one chemotherapy regimen over another, the quality of life associated with each chemotherapy regimen must be taken into account, besides the patients’ performance status, their lifestyle, age and education level.











 text in
text in