Introduction
Patients with persistent severe asthma comprise only 5% to 10% of the asthmatic population. However, compared to the general asthmatic population, these patients experience the greatest overall morbidity and impact on quality of life, and are associated with higher costs1. The problem of severe refractory asthma or difficult-to-control asthma has been addressed in the clinical practice guidelines Global Initiative for Treatment of Asthma (GINA)2and the Spanish Guidelines for Asthma Management (GEMA)3. However, a large percentage of patients are poorly controlled, undertreated, and without adequate follow-up, which is indicative of poor adherence to the guidelines and expert recommendations4. This situation reflects the current reality of asthma as a public health problem worldwide, which is one of increasing magnitude and prevalence2,3.
Omalizumab is an anti-IgE monoclonal antibody that prevents and reduces the release of pro-inflammatory mediators. It is indicated as an additional therapeutic modality in patients with severe persistent allergic asthma refractory to standard treatment. In the last decade, controlled clinical trials and “real life” studies have shown that omalizumab is effective and well tolerated in the treatment of moderate to severe asthma, particularly in the reduction of exacerbation rates and the use of inhaled corticosteroids5 6-7. In contrast to strong evidence on its efficacy and safety profile in the treatment of asthma, there is limited information on the cost-effectiveness of its use and the results of systematic reviews and meta-analyses remain inconclusive8,9.
In Spain, only two cost-effectiveness studies of omalizumab for the treatment of severe asthma have been published10,11. These studies were conducted using small samples of patients. One study included 47 patients treated in the Pneumology Service of the Hospital Universitario Virgen de la Victoria of Málaga (Spain)10and the other included 86 patients treated in the Pneumology Service of the Hospital Clínic of Barcelona (Spain)11. Both studies showed that treatment with omalizumab was cost effective as assessed by the reduction in asthma exacerbations and 3-point increases in the Asthma Control Test (ACT) score. The results were expressed as the incremental cost-effectiveness ratio. In order to confirm these findings in a larger sample of patients, we conducted a study in 12 Pneumology Services within the Valencian Community (Spain). The objective of the study was to assess the effectiveness of omalizumab for the treatment of patients with severe asthma, its impact on quality of life, and benefits regarding the reduction of direct and indirect costs under conditions of standard clinical practice.
Methods
Study design
Observational retrospective multicentre study conducted under conditions of standard clinical practice in 12 Pneumology Services in the Valencian Community. A retrospective review of medical histories was conducted in the participating centres from 2006 (i.e. the year in which omalizumab was first marketed in Spain) to April 2014. The study included all patients visiting the outpatient consulting offices of the Pneumology Service of the participating centres who met the following eligibility criteria: equal to or more than 18 years, diagnosed with severe asthma2,3(steps 5-6 of GEMA and 5 of GINA), and receiving omalizumab as an add-on therapy to standard treatment for at least one year prior to inclusion.
We obtained clinical data, treatment data, and data on the use of health care resources and work absenteeism for patients for the year prior to the addition of omalizumab (pre-omalizumab period) and for subsequent years (post-omalizumab periods). Although all the study patients had data available for the first year of treatment with omalizumab, which was an eligibility criterion, there was less data available for the post-omalizumab periods (years 2-5).
We assessed effectiveness of treatment, use of resources, and quality of life for each year of treatment with omalizumab. One-year periods were chosen to avoid any potential seasonal bias.
The study complied with the principles of the Declaration of Helsinki and the Good Clinical Practices Guidelines and was approved by the Clinical Research Ethics Committee of the Hospital Universitario Doctor Peset.
Measures of effectiveness, resources, and quality of life
The pre- and post-omalizumab periods were compared using the following indicators of effectiveness: the asthma exacerbation rate, the ACT score12, and the assessment of clinical asthma status by the physician (Global Evaluation of Treatment Effectiveness (GETE scale))13. Exacerbations were defined as a worsening of symptoms that required rescue treatment with systemic corticosteroids. The total number of exacerbations was calculated as the sum of prednisone cycles, number of visits to the emergency department, and hospital admissions due to asthma.
The assessment of direct costs included the following resources: a) number of unscheduled visits to hospital and primary care due to asthma exacerbation, b) number of visits to hospital and primary care emergency services due to asthma exacerbations, c) number of hospitalizations due to asthma exacerbation, d) days of hospital admission, and e) dose, dosage schedule, and duration of treatments.
The impact of omalizumab on the patients’ quality of life was assessed using the Asthma Quality of Life Questionnaire (MiniAQLQ) and European Quality of Life-5 dimensions (EQ-5D-3L) questionnaires.
Calculation of direct and indirect costs
The economic impact of additional omalizumab for the treatment of severe asthma was assessed using direct costs (use of health care and pharmacological resources) and indirect costs (impact of the disease on labour productivity)14. The total cost of pharmacological treatment for each patient was calculated using the dose, the dosage schedule, the duration of each treatment, and the associated unit cost. The unit costs of treatment were calculated using the corresponding data published on the Spanish General Council of Official Associations of Pharmacists website, applying the Royal Decree-Law 8/2010 (RDL 8/2010) deduction on the recommended retail price (RRP) without VAT for ambulatory dispensing treatments and the corresponding RDL 8/2010 deduction on the laboratory sale price (LSP) for hospital dispensing drugs. The cost of omalizumab was calculated by applying the RD deduction of 7.5% on the LSP. Given that omalizumab is administered in hospitals, the cost of one nursing visit per month was added when omalizumab was administered once per month, and the cost of two nursing visits per month was added when it was administered once every two weeks. We calculated the annual costs of the use of resources as the product of the natural units of the resources used at the end of one year and their associated unit cost. The unit prices of the resources were obtained from the eHealth database15. Indirect costs were calculated based on the human capital method, by assuming that wages reflect worker productivity14. The days that the patient could not go to work due to asthma (absenteeism) were multiplied by the daily wage cost specific to sex and age. The wage costs were extracted from the most recent data published by the Spanish National Institute of Statistics. These data were obtained from the Spanish Annual Wage Structure Survey 2013. All costs were expressed in 2015 euros.
Cost effectiveness analysis
A cost-effectiveness analysis was performed to investigate the impact of omalizumab on costs and clinical outcomes. The incremental cost-effectiveness ratio (ICER) was calculated by comparing costs and effectiveness in the pre- and post- omalizumab periods in terms of prevented asthma-driven exacerbation and 3-point increases in the ACT score.
Cost-utility analysis
A cost-utility analysis was conducted to measure the health benefits of the treatment in quality-adjusted life years (QALY). The incremental cost-utility ratio (ICUR) was calculated in terms of the increase in QALYs.
Statistical analysis
In the descriptive analysis, categorical variables are expressed as frequencies and percentages, and quantitative variables are expressed as mean and standard deviation or the median plus the interquartile range.
The main statistical analysis compared the clinical results and the use of resources in the pre- and post-omalizumab periods, in which each year was compared with the immediately previous year. We conducted descriptive bivariate tests on all the study variables, parametric or nonparametric bivariate tests according to their applicability (comparison of means: t test, Wilcoxon signed rank; contingency tables: χ2 test, Fisher’s exact test, Mc-Nemar test; and correlations: Pearson, Kendall tau, Spearman rho). In order to increase statistical power, whenever possible the tests used took into account paired observations using pre- and post-omalizumab data for all patients. A P -value of 5% was used as a cutoff for statistical significance. The results of the ICER/ICUR calculations are given with their 95% confidence intervals (95% CI), which were obtained using the bootstrap technique. All statistical analyses were conducted using the R version 3.1.2 statistical software package.
Results
A total of 186 patients were eligible for inclusion in the study. Of these, 62.4% were men and mean age was 50.5 years (95% CI: 48.2-52.7). 8.1% of the patients were active smokers and 15.1% were ex-smokers. Median time from the diagnosis of asthma to starting treatment with omalizumab was 15 years (interquartile range: 6-24). The main comorbidities were rhinitis, nasal polyposis, and respiratory allergy in 60%, 30.5%, and 59.2% of patients, respectively.
Effectiveness
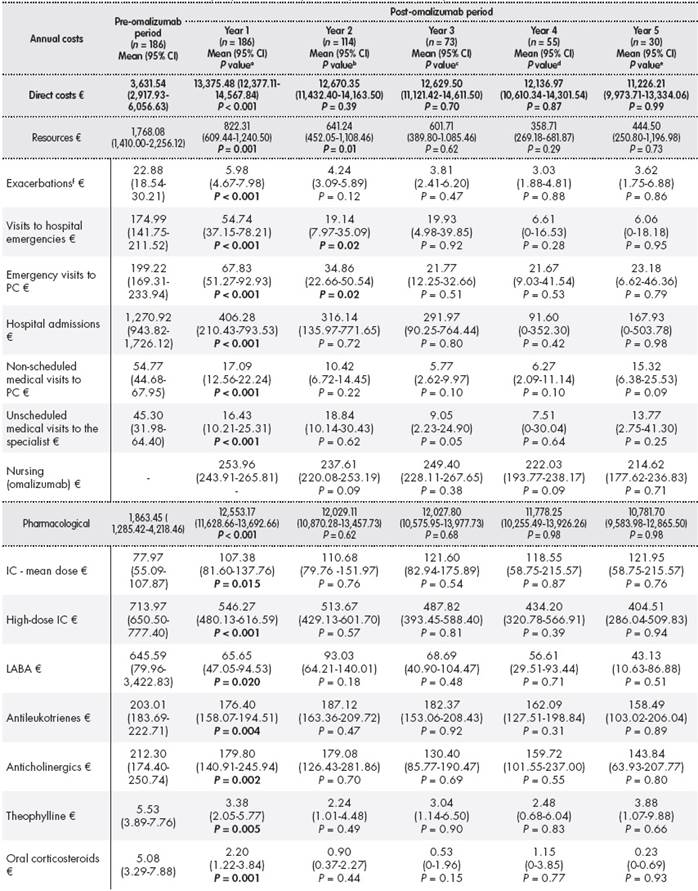
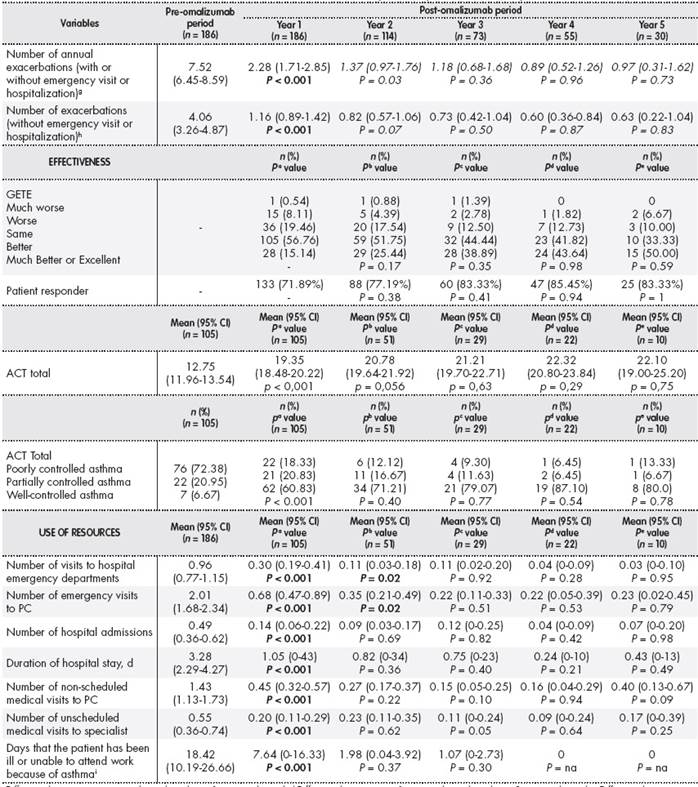
The addition of omalizumab to the standard treatment during the first year of treatment led to a decrease in the ACT score from 12.75 points (95% CI: 11.96-13.54) in the pre-omalizumab period to 19.35 points (95% CI: 18.48-20.22) in the post-omalizumab period (P < 0.001). The ACT score showed that the percentage of poorly controlled patients decreased from 72.38% to 18.33% ( P < 0.001). Treatment with omalizumab led to a statistically significant decrease in the number of annual exacerbations (7.52 (95% CI: 6.45-8.59) vs 2.28 (95% CI: 1.71-2.85); P < 0.001). The GETE scale showed that 71.9% of patients had a better or much better response with omalizumab. A statistically significant increase was observed (P < 0.001) in spirometric FVC parameters (80.96% (95% CI: 78.16 -83.75) vs 87.68% (95% CI: 84.95 - 90.41)), post-bronchodilator FEV1 (67.29% (95% CI: 64.15-70.42) vs 75.23% (95% CI: 72.30-78.16)), and FEV1/FVC (64.42% (95% CI: 62.35-66.50) vs 67.10% (95% CI: 65.01-69.20)). The results obtained in the four years following the first year of treatment with omalizumab were similar to those obtained in the first year of treatment (Table 1 ). However, after the first year of treatment with omalizumab, the patients perceived that their asthma had improved or had greatly improved (83.9%). In addition, the percentage of patients with poor or bad control of rhinitis decreased from 35.48% to 2.15% (P < 0.001).
Table 1. Clinical variables, effectiveness, and use of resources in the pre- and post-omalizumab periods
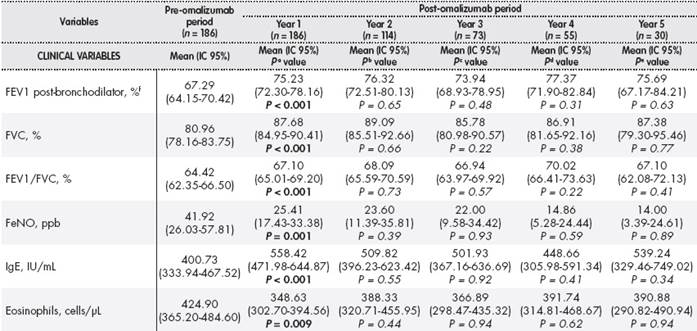
Table 1(cont.). Clinical variables, effectiveness, and use of resources in the pre- and post-omalizumab periods
aDifference between year pre-omalizumab and year 1 post-omalizumab;bDifference between year 1 post-omalizumab and year 2 post-omalizumab;cDifference between year 2 post-omalizumab and year 3 post-omalizumab;dDifference between year 3 post-omalizumab and year 4 post-omalizumab;eDifference between year 4 post-omalizumab and year 5 post-omalizumab;fPercentage relative to theoretical FEV1;gIncreased symptoms with or without hospitalization requiring treatment with systemic corticosteroids;hWorsened symptoms requiring treatment with systemic corticosteroids without hospitalization;in = 90 patients in pre-omalizumab period; n = 88, 49, 29, 25 and 11 patients in years 1, 2, 3, 4, and 5, respectively.
ACT: Asthma Control Test; CI: confidence interval; FeNO: expiratory fraction nitric oxide; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; GETE: Global Evaluation of Treatment Effectiveness; IgE: immunoglobulin E; na: not assessable; PC: primary care; ppb: parts per billion; UI: international unit.
Statistical tests: χ2test for categorical variables, and paired Mann-Whitney U test for quantitative variables, and nonparametric paired Mann-Whitney U test.
One year after starting treatment with omalizumab, 13 patients (6.95%) had discontinued treatment. Of these, eight patients stopped treatment for their own reasons, three stopped because of lack of response, and two stopped because of adverse effects (one because of nausea and vomiting and one because of itching).
Use of resources
The addition of omalizumab led to significant reductions in the number of patients with prescriptions for high-dose inhaled corticosteroids (HDIC), anticholinergics, theophylline, and oral corticosteroids (OC). There was also a significant reduction in the mean dose of HDIC (1,006.4 vs 752.1 μg/d; P = 0.004), long-acting ß2 adrenergic receptor agonists (LABA; 96.1 vs 58.7 μg/d; P < 0.000), antileukotrienes (7.7 vs 6.9 mg/d; P = 0.008), anticholinergics (72.4 vs 6.4 μg/d; P < 0.000), theophylline (70.1 vs 43.6 mg/d; P < 0.000), and OC (1.7 vs 0.8 mg/d; P = 0.001). During the first year of treatment, the mean dose of omalizumab per patient was 419.8 ± 259.86 mg/mo.
Treatment with omalizumab led to statistically significant decreases in the number of hospital admissions (0.49 (95% CI: 0.36-0.62) vs 0.14 (95% CI: 0.06-0.22); P < 0.001), the number of unscheduled medical visits, and emergency visits to both primary care and hospitals. The mean time of hospital stay decreased from 3.28 days (95% CI: 2.29-4.27) to 1.05 days (95% CI: 0.35-1.75) (P < 0.001). This decrease in the use of resources remained practically constant during the following four years, except for the number of emergency visits between the first and second year (Table 1 ).
Quality of life
After the first year of treatment with omalizumab, there was a clinically and statistically significant increase in quality of life on the four domains of the MiniAQLQ questionnaire and its overall score (3.26 points (95% CI: 2.88-3.63) vs 4.92 points (95% CI: 4.42-5.43); P < 0.001). Statistically significant improvements were also observed on the four dimensions of the EQ-5D questionnaire and its visual analogue scale (47.96 points (95% CI: 43-52) vs 70.33 points (95% CI: 64-75); P < 0.001). During the first year of treatment with omalizumab, there was also a significant increase in QALYs from 0.61 (95% CI: 0.54-0.68) to 0.81 (95 CI: 0.74-0.88) (P < 0.001) (Table 2).
Table 2. Results of the EQ-5D Questionnaire and QALYS in the pre-omalizumab period and the first year post-omalizumab

EQ-5D: European Quality of Life-5 Dimensions; QALYS: Quality-ajusted life years.
Statistical tests: χ2test for categorical variables and paired Mann-Whitney U test for quantitative variables.
Costs
A statistically significant decrease was observed in the mean annual cost of resources used between the pre- and post-omalizumab periods (€ 1,768.08 (95% CI: 1,410.00-2,256.12) vs € 822.31 (95% CI: 609.44-1,240.50); P = 0.001). There was a decrease in the annual cost of exacerbations, visits to the emergency room, hospital admissions, and unscheduled medical visits.
In the post -omalizumab period, there was a significant decrease in the annual pharmacological costs derived from the use of HDIC, LABA, antileukotrienes, anticholinergics, theophylline, and OC. The mean cost of omalizumab per patient/y was € 11,472.14 (95% CI: 10,513.52-12,536.62). In the post-omalizumab period, there was also a decrease in the mean indirect cost per patient from € 714.95 (95% CI: 459.28-1,160.46) to € 398.42 (95% CI: 155.12-958.43) (P < 0.001). In the four years following the first year of treatment with omalizumab, there were nonsignificant reductions in direct and indirect costs (Table 3 ).
Table 3(cont.). Annual direct and indirect costs per patient in the pre- and post-omalizumab periods
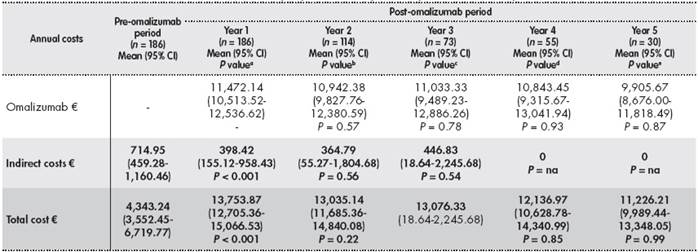
aDifference between year pre-omalizumab and year 1 post-omalizumab;bDifference between year 1 and year 2 post-omalizumab;cDifference between year 2 and year 3 post-omalizumab;dDifference between year 3 and year 4 post-omalizumab;eDifference between year 4 and year 5 post-omalizumab;fWithout visit or hospitalisation.
CI: confidence interval; IC: inhaled corticosteroids; LABA: long-acting χ2adrenergic receptor agonists; na: non-assessable; PC: Primary care.
Statistical tests: nonparametric Mann-Whitney U test.
Analysis of the cost-effectiveness and cost-utility ratio
In terms of direct costs, the ICER per exacerbation avoided was
1,858.84 (95% CI: 1,084.72-3,137.62) and the ICER per each 3-point increase in the ACT score was € 4,737.42 (95% CI: 3,590.60-6,208.86). The ICUR per QALY was € 53,755.34 (95% CI: 40,663.14-72,497.39). When including indirect costs, the ICER per exacerbation avoided was
1,858.84 (95% CI: 1,084.72-3,137.62) and the ICER per each 3-point increase in the ACT score was € 4,737.42 (95% CI: 3,590.60-6,208.86). The ICUR per QALY was € 53,755.34 (95% CI: 40,663.14-72,497.39) (Table 4).
Table 4. Incremental cost-effectiveness and incremental cost-utility ratio including direct and total costs
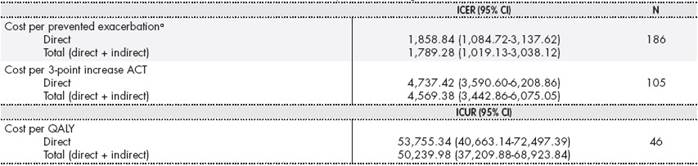
aExacerbation with or without emergency visit or hospitalization.
ACT: Asthma control test; CI: confidence interval; ICER: incremental cost-effectiveness ratio; ICUR: incremental cost-utility ratio.
Discussion
The efficacy and favourable safety profile of omalizumab as an add-on therapy to HDIC and LABA in patients with severe uncontrolled asthma has been widely documented in the literature16 17 18-19. The present study showed that after 12 months of treatment with omalizumab there was a mean increase of 6.6 points in the ACT score (51.8% of patients) and a mean reduction of 5.2 exacerbations per year (69.7% of patients) compared to the pre-omalizumab data. These results are similar to those obtained in clinical trials and observational studies showing that treatment with omalizumab is associated with a relative reduction in the number of exacerbations from 25% to 75%13,16 17-18,20and an increase in the ACT score in 41.9% to 60% of patients13,18,21. The favourable results on improvements in quality of life are also similar to the results of previous studies18,22. Specifically, we observed improvements in the MiniAQLQ scores (a 1.66-point increase in 50.9% of the patients) and on the four dimensions of the EQ-5D, as well as a significant increase of 0.20 QALYs (32.8% of patients) during the first year of treatment.
Most of the studies that have assessed the pharmacoeconomic impact of omalizumab and its impact on the use of resources have either been conducted using small samples of patients10,11,23or have based their results on efficacy data obtained in clinical trials24,25. In contrast, this multicentre pharmacoeconomic assessment study is the first to be conducted under clinical practice conditions in Spain that has included a large number of patients. The most relevant results include an ICER of € 1,789.28 for avoided exacerbation and € 4,569.38 for a 3-point increase in ACT scores.
Previous studies in this field have reported ICERs of between € 462 and € 1,131 for exacerbation avoided and € 4,124.79 for a 3-point increase in ACT scores10,11. Several studies have reported costs of between € 23,880 and € 56,091 for each QALY gained10,24,26. The latter value is similar to that obtained in this study (€ 50,239.98).
It has been suggested that 22% of the costs associated with severe uncontrolled asthma can be attributed to indirect costs27). In this sense, the results of the present study also show a decrease in indirect costs (€ 316.53) and in direct costs derived from exacerbations, visits, and hospital admissions (€ 945). This study is the first to analyse the pharmacoeconomic impact of omalizumab in the medium term. After the first year of treatment, clinical evolution, resource use, and costs remained practically constant over the next four years. This result could be interpreted as being due to the sustained clinical effectiveness of omalizumab and its medium-term efficiency.
Two of the limitations of the present study are the lack of clinical records beyond the first year of treatment with omalizumab and the small number of patients (n = 46) on which the cost-utility analysis was based because of the lack of retrospective data on quality of life. Levy et al.10have previously described an ICUR of € 26,864.89 per QALY in relation to direct costs alone. However, we calculated the cost per QALY gained with omalizumab while taking into account the indirect costs. These costs were calculated based on the human capital method, which assumes that the wages reflect worker productivity14. The wage costs were extracted from the most recent data published by the Spanish Institute of Statistics. It should be noted that the external validity of the study may not be applicable to the health systems of other countries with different organizational characteristics.
In summary, the results obtained in this study suggest that, in addition to sustained clinical improvements and increases in the number of QALYs, patients treated with omalizumab require less healthcare, consume fewer health resources, and entail lowered costs because of decreased absenteeism. These results are of clinical relevance and should be taken into account in daily practice when choosing an effective and cost-efficient treatment for patients with severe asthma.











 text in
text in