Introduction
Patients’ safety in healthcare systems is a priority area and hence is reflected in the National Health System’s 2015 -2020 Patient Safety Strategy. Its objectives are focused on improving safety practices, risk management in healthcare, training and the involvement of patients-citizens1.
The frequency of adverse events in hospitalised patients in the Europen Union (EU) is 8-12%, i.e., 1 death/100,000 inhabitants/year, which represents about 5,000 deaths/year2.
In Spain, a number of studies have analyzed the inadequacy of the system in several healthcare areas. More precisely, ENEAS: a study of the adverse events related to hospitalisation, using a cohort of 5,624 inpatients from 24 Spanish hospitals, estimated that the incidence of patients with adverse healthcare-related events was 9.3% in one day. Of these events, 37.4% was related to medication3. Another study revealed that 53% of EU citizens reckoned that they could be potentially harmed when receiving hospital care. In Spain, 11.5% of respondents to the 2010 Health Barometer reported having suffered from medical error during hospital stay4.
All these events have an important socio-economic impact when one takes into account that they could be avoided 50-70% of the time and that they generate an additional annual budget expenditure of 5%. In the particular case of medication provided to inpatients, a 2011 study estimated that the cost of avoidable events to the National Health System (NHS) would be around € 1,779 million5.
This was the turning point on this subject. Many organisations like the Agency for Healthcare Research and Quality, the National Quality Forum, the Joint Commission and the World Health Organisation are working towards improving patients’ safety. Their strategic lines establish working guidelines for implementing “safe practice standards”. All of them lay particular emphasis on high-alert medications since they have a greater possibility of association with serious or fatal events. These organisations highlight that health institutions should identify such medications and establish procedures for their safe use in healthcare procedures1.
The Institute for Safe Medication Practices (ISMP) has issued a classification of high-alert medications as well as the risk factors associated with their administration such as staff turnover, temporary jobs, training, identification, etc., and has in addition elaborated a guide to improve clinical practice when handling such medications6.
Ever since the 1950s and in a number of non-health related areas, the development and management of risk maps have been used as informative tools, which by means of descriptive information and appropriate indicators, facilitate the identification of certain activities or procedures subject to risk, and furthermore quantifies the probability of occurrence of such events and measures any associated potential harm7,8.
Therefore, the objectives of this study are:
Methods
An observational and descriptive six month study was carried out in a high level University Hospital (with 1,200 beds and in area of 600,000 inhabitants). The scope of the study included adult clinical units at the hospital. It was developed in two phases:
1stPhase: “Design and development of a risk map relating to usage of high-alert medications”
A multidisciplinary group was first created (six pharmacists, two professionals from the quality assurance department and three consultants from the clinical-healthcare field: two doctors, one nurse). In a first stage, structured surveys were carried out to assess the opinion and knowledge of other professionals on high-alert medications.
A bibliography search was then conducted through PubMed on the handling of high-alert medications and the elaboration of risk maps. This information was used to identify the structure of the map9and the severity levels of the high-alert medications. The high-alert medications from the Pharmacotherapy Guide were then identified using the ISMP6,10classification as reference.
This information was used to define the criteria and factors that could bear a higher incidence when handling high-alert medications:
Location: The number of high-alert medications handled (by location) in nursing units with adult inpatients (not performed by service since there were several services in one nursing unit).
Staff turnover (ST): The high percentage of staff rotation in units requiring specialisation was considered to have a higher potential risk6. Data provided by the Human Resources Department was used to assess and classify units into three major groups: low rotation = 1 point (average ratio-1 standard deviation), intermediate rotation = 2 points (average ratio ± 1 standard deviation), and high rotation = 3 points (average ratio medium + 1 standard deviation).
Frequency of event (FE): The occurrence of an event was considered to be related to the amount of high-alert medications handled in each unit. The rate of use of high-alert medications was defined as the coefficient obtained upon dividing the number of high-alert medication units used in each clinical unit by the total number of medications used in that same unit. Units with below average ratio -1 standard deviation were considered as low-risk (1 point), those with an average ratio + /-1 standard deviation were considered as medium risk (2 points), and units with an above average ratio + 1 standard deviation were classified as high risk (3 points).
Severity differences between high-alert medications: the lack of information on severity differences between high-alert medications events led us to survey medical and nursing staff, in order to weight in the severity of a possible high-alert medications adverse event, according to their perceptions in daily professional practice. It was voluntary, anonymous and targeted at professionals from different areas (medical, surgical and critical care units), in order to ensure that the final results were representative.
Ten doctors and 26 nurses from the medical, surgical and critical care services that participated in the survey. Each high-alert medication was weighted 1-3 according to an average severity score (1 = significant; 2 = serious; 3 = life- threatening). To obtain the final severity score, the average value was calculated taking into account the number of professionals who responded in each column and the severity they indicated. For example, a score of 20 corresponding to the Intravenous Contrast Agents = ((3 ?? 1) + (12 ?? 2) + (11 ?? 3)) / 3 (Table 1).
Table 1. Severity score of the different high-alert medications as per the score obtained in the perception survey of health professionals
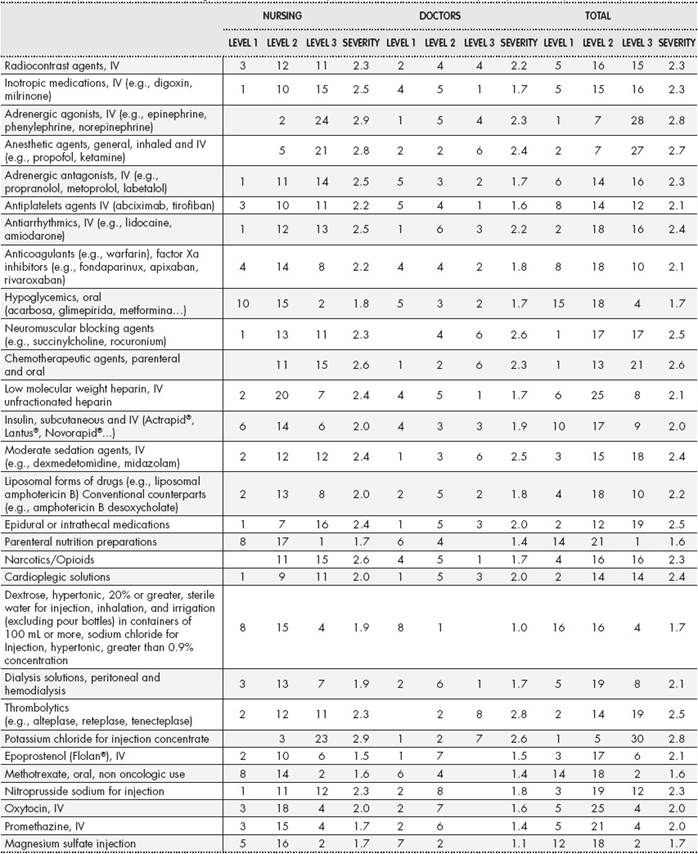
IV: intravenous.
A cumulative risk (CR) of severity for each unit was calculated using the formula: ∑ (Severity of medication x no. of medication units). The natural (Napierian) logarithm was applied to this score to reduce variability of values.
This was used to establish the risk probability index (RPI) = Staff Rotation (SR) x FE x LnCR.
For the descriptive analysis of the results the mean deviation standard was calculated in the case of the quantitative variables data, as well as the absolute and relative frequency in the case of the categorical variables.
2ndPhase: “Classification of hospitalisation units by risk”
The above parameters were used to classify units into three groups by assigning a colour code: high risk-red; medium risk-yellow; low risk-green.
Targeted risk prevention and training actions and strategies were then established in line with the risk map obtained6,10.
Results
A total of 447 high-risk medications corresponding to 227 active ingredients were identified during the study period. The number of high-alert medications dosages dispensed was 195,287. Risk factors were assessed in 25 nursing units from 30 services.
The units that reported a greater use of high - alert medications as compared to the total number of medications used in the study period were Haematology (34.10%), Intensive Care Medicine (30.02%), Oncology (20.12%) and Reanimation (19.03%).
The severity survey results of the potential adverse events are shown inTable 1. Doctors indicated that thrombolytics/fibrinolytics, neuromuscular blocking agents and intravenous potassium can produce a higher severity event, while the nurses considered intravenous adrenergic agents, intravenous potassium and general anaesthetics as medications that can produce such an event. When asked what they believed could cause a high-alert medication event, 40.3% of the respondents mentioned dosage errors, 25.7% indicated lack of training or competence of staff high-alert medications, 18.2% thought that it could be caused by an incorrect administration route, while 15.8% felt that the type of population could determine the occurrence of a high-alert medication event.
The final map (after taking into account the factors described above) is shown inTable 2. Actions for improvement were agreed upon in accordance with the RPI risk number obtained, and they are described inTable 3. Low risk measures were applied to clinical units with RPI < 1, medium risk measures to units with RPI between 1-2.9 and high-risk measures to units with RPI > 2.9.
Table 2. Event-probability risk map on the use of high-alert medications by hospitalization unit

ANR: anaesthesia and reanimation; AVS: angiology and vascular surgery; CAR: cardiology; DT: digestive tract; END: endocrinology; ENT: otorhinolaryngology; GDTS: general and digestive tract surgery; GER: geriatrics; GYN: gynaecology; HAM: high alert medication; HAEM: haematology; LN: neperian logarithm; ICC: intermediate care cardiology; ICM: intensive care medicine; ICS: intermediate cardiac risk surgery; ICU: stroke unit; IMa, IMb: internal medicine; Med: medication; NEF: nephrology; NRL: neurology; NRS: neurosurgery; OBS: obstetrics; ONC: oncology; ORT major: orthopedic major surgery; ORT minor: orthopedic minor surgery; PCU: palliative care unit; PS: plastic surgery; PSY: psychiatry; PUL: pulmonology; RDT: radiation therapy; REH: rehabilitation; RHEUM: rheumatology; RPI: risk probability index; SSU: short stay unit; TS: thoracic surgery; URO: urology.
Discussion
To the authors’ knowledge, no similar study has been published to date and hence this work represents an innovative analysis model to address prevention strategies linked to high-alert medication errors in hospitals. Risk maps have exceptionally been used in the healthcare field and have always been focused on improving organization11.
The creation of a risk map permits assessment and knowledge of the risks inherent to each clinical unit in order to develop a structured corporate safety plan for high-alert medications in hospitalised patients based on real needs. This study has provided us with an insight not only into healthcare service provision relating to high-alert medications in a large hospital but also information on which units we need to establish more prevention measures in. These measures nevertheless follow the standards laid down by international safety bodies6,7.
The criteria agreed upon to develop the map are not entirely novel. High staff turnover, temporary jobs, and the amount of high-alert medications have been recognised as key special action points for the prevention of errors1,6. Nevertheless, to ascertain the influence of event severity in terms of whether error is due to one or more high-alert medications12or due to lack of training13, has been difficult to establish, as there are no standardised classification methods in this regard. Engels et al. (2015) presented a study carried out in the University of Michigan Health System (based on the use of surveys to determine training level and perceptions of high-alert medications), in which they concluded that the perception of high-alert medications differed depending on professional profile, and that specific educational measures needed to be implemented in each of them13. This conclusion is also reached in the present study, and therefore improvement strategies suggest the development of a tailored and targeted training itinerary.
Highest risk units detected in the study are similar to those documented in the bibliography section.
Critical care units are the ones at highest risk due to the amount of high-alert medications they handle and also in line with the results of this study, due to high staff turnover. It is striking to note that plastic and orthopedic surgery, which initially could be considered as low-risk units, are in fact at the top of the classification. This can be justified because of the large amount of low molecular weight heparins administered and the high staff rotation levels present.
The effectiveness of strategies for the prevention of medication administration errors with high-alert medications has been the subject of scientific study for years now. Examples are the use of smart infusion pumps14or the reconciliation of medication in Emergency Departments15, and the implementation of administration protocols for high-alert medications in certain units. Additionally, Cuesta López et al. (2016) also analysed effectiveness in relation to vasoactive drugs in critical care units16. However, an analysis prior to overall handling of high-alert medications or the optimisation of measures to be implemented is hardly ever performed.
The main limitations of the study have been that we have not validated or calculated the sample size of the surveys on the potential severity of an error involving high-alert medications. Nevertheless, large prospective multi-centre studies in hospitals of similar characteristics could improve this aspect.
In conclusion, this study describes a method of analysis to know how high-risk medication is managed in a high level hospital. The risk map allows us to detect, from a multidisciplinary approach, which are the most critical hospitalisation units. This makes it possible to prioritise which of them need more control and training measures on High alert medications.















