Introduction
Empyema is a complicated parapneumonic pleural effusion (PE) defined by the presence of pus and/or microorganisms in the pleura. It is characterized by a higher concentration of proteins than plasma (DE proteins/plasma proteins ratio > 0.5 g/L), high lactate dehydrogenase (> 1,000 U/L) and an acidic pH (< 7.2) as a result of the presence of pus in the pleura1-3. The basis of its treatment consists in the placement of a pleural drainage for the evacuation of pus along with a systemic antibiotic treatment1,3,4. However, the lack of empyema resolution with the standard therapy is related to the presence of high amounts of fibrin in the infected pleural space, and high-density partitioned spaces that prevent the correct purulent material drainage, which also blocks the effectiveness of systemic antibiotic therapy (example shown in Figure 1). Hence the interest in intrapleural fibrinolytic therapy.

Figure 1. Ultrasound image corresponding to empyema. D: diaphragm; H: liver (“hígado” in Spanish); P: pulmonary parenchyma. Arrows indicate the multiple fibrin partitions within the pleural effusion.
Over the past 15 years, several studies have evaluated the efficacy and safety of fibrinolytic therapy, although results are not entirely conclusive. A first study -the MIST1 study-5, showed that intrapleural administration of streptokinase did not improve either mortality, the need for surgery, or the length of hospital stay. Subsequently, based on the experience of using deoxyribonuclease (DNase) to reduce the viscosity of secretions in patients with cystic fibrosis, it was postulated that deoxyribonucleic acid (DNA) was the main cause of the viscosity increase in the pleural space. This fact generated the hypothesis that intrapleural administration of the DNase enzyme improves the efficacy of pleural drainage. It was in 2011 when Rahman et al.6 proved, in a large randomized controlled study, an improvement in the response to parapneumonic pleural effusion therapy (PPE) empyema type by combining alteplase and dornase alfa, achieving a marked and significant reduction in the days of mean hospital stay, as well as of the need for surgical debridement of empyema compared with placebo, and also with respect to dornase alfa or alteplase in monotherapy. The doses used were empirically chosen, based on observational studies and previous case series6. The fibrinolytic drugs used differ between studies, using both non-specific fibrinolytic drugs -such as streptokinase and urokinase- as specific fibrinolytics -such as alteplase-. However, there are no comparative studies between them.
It is necessary to highlight that, to date, the most common fibrinolytic therapy guidelines in this field have not incorporated this combined strategy. This may be due to the lack of applicable protocols for use in clinical prac-tice that support both the feasibility of administering a combined intrapleural treatment, and the reduction of its complexity. Recent studies7 have tried to simplify the treatment with the concurrent intrapleural administration of both drugs. That is, without leaving a time of action between drugs as previously performed6. Although the authors conclude that the regimen is relatively safe and effective, the stability of the co-administration of both drugs has never been studied.
For this reason, the objective of this study is to analyze the physicochemical stability of the fibrinolytic agent and dornase alfa joint administration for the subsequent development of a clinical practice use protocol for PPE patients that require intrapleural treatment.
Methods
Dose preparation
A stock solution of 500,000 IU of urokinase dissolved in 10 mL of normal saline (0.9% sodium chloride, NS) and a stock solution of 10 mg of dornase alfa 2.5 mg/2.5 mL in 60 mL of water for injection (WFI) was prepared. Since these drugs, when used for empyema therapy, are employed in a 1:6 ratio (250,000 IU of urokinase in 5 mL of NS versus 5 mg of dornase alfa in 30 mL of WFI)6, we used 1 mL of the urokinase solution stock and 6 mL dornase alfa stock solution to form three aliquots of the combination of the two drugs (7 mL) to carry out the stability study in triplicate.
In vitro stability analysis
Stability was defined as the absence of particles, color variation or changes in the pH mixture. Samples were subjected to: (i) a visual examination to detect precipitation, (ii) a centrifugation process for the detection of precipitates not observed by sight (iii) and a pH measurement (related to temperature and protein denaturation).
Regarding the visual examination, two different observers intervened to evaluate color, cleanliness, bubble formation and/or precipitate. PH measurement was performed with a PH BASIC 20 Meter (CRISON, Barcelona) and centrifugation was performed in a Centromix II -BL digital Centrifuge (JPSELECTA, Barcelona). The test tubes containing the mixture were maintained at physiological temperature (37 °C) in a thermostatic bath (LACOR, Bergara). All these instruments were calibrated according to the corresponding standardized work procedure by the Pharmacy Service Laboratory, prior to the measurements.
Observations were carried out at time 0 (baseline), 30 minutes, 1, 2 and 4 hours from the mixing process.
Results
The results of the physicochemical analysis, shown in Table 1, showed that the stock solution of urokinase is slightly acidic (pH = 6.95 ± 0.04), while that of dornase alfa is neutral (pH = 7.32 ± 0.01). However, the mixture of both drugs had a pH of 5.63 ± 0.02.
Table 1. Results obtained in the in vitro analysis of urokinase, dornase alfa and the combination of both drugs

Pellet: each portion of sedimented material; SD: standard deviation.
Regarding the time of joint action of the studied enzymes, a change in the stability of the samples was clearly observed. At 30 minutes and 1 hour times, the samples did not show turbidity, and their centrifugation did not cause pellets (portions of sediment material), while at 2 and 4 hours of contact of both enzymes, a formation of flocs was observed (Figure 2). After 4 hours at rest, separation of the combination into two phases occurred, with the consequent formation of precipitate.
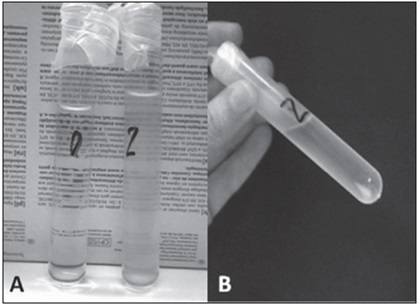
Figure 2. A) Mixture of dornase alfa and urokinase at time 0 (clear) and at 2 hours (turbid). B) The pellet formation (arrow) that occurs after centrifugation of the test tubes subjected to 2 and 4 hours of contact between the drugs studied.
From the results obtained, an action protocol was developed based on a sequential administration of the drugs, as detailed inTable 2. This would firstly consist in the administration of 100,000-250,000 IU of urokinase, while maintaining a 2 hour clamping time. After this period of time, urokinase would be removed and 5 mg of dornase alfa would be administered with a clamping time of 2 more hours. The administration of intrapleural drug treatment would be maintained for 3 days with the administration of urokinase at a frequency of every 8 hours and of dornase alfa every 12 hours.
Discussion
Results of the in vitro evaluation indicated that it is not possible to guarantee stability, and therefore ensure the pharmacological activity of urokinase and dornase alfa after 2 hours of mixing both drugs. Hence, the possibility of simultaneous or concurrent administration of both drugs in clinical practice was ruled out. It was then decided to develop an action protocol based on the sequential urokinase and dornase alfa administration.
Data regarding the possibility of the joint use of urokinase and dornase alfa are contradictory, since in the dornase alfa summary of product characteristics it is specified that it can be administered safely and effectively together with other drugs8. However, joint use of urokinase with other drugs is discouraged9. In addition, previous studies, despite demonstrating comparable in vivo efficacy between the administration of both drugs sequentially and simultaneously7, do not evaluate the stability of the mixture6,7, which does not allow clarification of this matter. To our knowledge, our study is the first to prove the physicochemical instability of the mixture of the two active ingredients, clarifying the doubts existing so far.
The initial pH determination of the resulting mixture of the two enzymes was lower than expected (5.63 ± 0.02), taking into account that the minimum pH value of the individual components was 6.95 ± 0.04. One possible explanation could be that urokinase may have caused a dornase alfa lysis. Another hypothesis was that the decrease in pH was due to the fact that dornase alfa is less stable in NS, where the urokinase was diluted. To test this hypothesis, the pH of the dornase alfa solution in NS was measured, being 5.8 ± 0.04. From these results, we conclude that dornase alfa seems to lose stability in NS. Therefore, urokinase should be WFI diluted in order to analyze the stability of the mixture in a more favorable environment for the dornase alfa activity.
In our area, protocols for intrapleural administration of fibrinolytics used to date included the administration of urokinase every 8 hours, proceeding to close the drainage for a period of 4 hours, enabling the drug to act. Considering that the urokinase stability time also does not guarantee greater efficiency in more sustained periods of application, and that 2 hours of exposure are sufficient to achieve the desired fibrinolytic effect9, it was decided to reduce the clamping time to 2 hours. It is noteworthy that lower clamping times up to only 1 hour have demonstrated the same effectiveness6. Therefore, our proposal should be considered even as conservative. Dornase alfa 5 mg would be added to this therapy in such a way that it would be administered 2 hours after administration of urokinase, and once it was removed, in a 12-hour interval.
There is no level of evidence that justifies the intrapleural use of fibrinolytic agents as the unique local treatment of empyema. This fact is still surprising, considering the traditional and regular use of these agents and their high cost. On the other hand, there is first- level scientific evidence in favor of including dornase alfa in the therapy, despite it not being the indication approved in the summary of product characteristics10. It seems very appropriate to include this second drug, considering the use of two enzymatic therapies with different targets, in intrapleural treatment protocols when considering that the increase in economic cost is minimal. As a guideline, and taking into account the average estimated cost in the current year, monotherapy with urokinase during 3 days of treatment involves a direct cost (manufacturer price) of 685 euros per treated patient, while combined treatment with dornase alfa assumes an additional cost (manufactured price) of 247 euros. This cost difference is largely offset by the expected reduction in days of stay and, especially, by the decrease in indications for surgical debridement, since patients without intrapleural therapy are almost six times more likely to require surgical treatment than patients undergoing intrapleural therapy with fibrinolytics and dornase alfa6. It is also necessary to emphasize that avoiding chest surgery not only implies a cost benefit, but also implies less general morbidity, avoids postoperative pain and other aspects that are not highly valued, such as the stress associated with passing through the operating room. Therefore, the potential benefit of double enzyme therapy is also significant from the patient's point of view.
Moreover, this study has some limitations that should be taken into account. A first limitation arises from the conditions in which the in vitro analysis has been carried out, which differs from those of the medium in which these drugs will act. The viscosity increases the stability by hindering the movement of the particles and, therefore, their aggregation as well. Thus, it is possible that the mixture is marginally more stable in the place of action that is rich in pus. Finally, this study was carried out with urokinase, as it is the most common used fibrinolytic in our center for this indication. Therefore, extrapolation of the results obtained to the rest of fibrinolytics will not be possible.
More studies are needed to clarify these questions. Even so, this analysis is a practical approach to the sequential intrapleural empyema therapy with both drugs, focussing on guaranteeing their physicochemical stability as a necessary step to obtain the best possible results in clinical practice.
In conclusion, we believe that there is very strong evidence in the literature to resort to double therapy with fibrinolytics and DNase to treat empyema. The data obtained in this stability study do not allow ensuring the simultaneous administration of both drugs in a safe and effective way, so we propose a sequential administration.











 texto en
texto en 



