Introduction
HIV infection is currently regarded as a chronic disease thanks to the extraordinary reduction in mortality achieved following the introduction of highly active antiretroviral treatment (ART) and the subsequent advent of more powerful and easy-to-manage therapeutic regimens. Increased survival has resulted in increasing numbers of patients reaching old age1. Estimations suggest that, by 2030, 73% of HIV patients will be over 50 years of age2. Similarly to the general population, ageing in HIV patients leads to the development of concomitant conditions. These comorbidities have been shown to appear earlier in patients living with HIV than in the general population3,4. An analysis of the DAD cohort (Data Collection on Adverse Events of Anti-HIV Drugs)5 demonstrated a prevalence of 33% of hyperglyceridemia, 22% of hypercholesterolemia, 8% of hypertension and 3% of diabetes mellitus, among other comorbidities6.
The development of concomitant conditions is associated to an increased use of drugs. Several studies have shown that the number of concomitant medications increases significantly after the age of 507,8. This increase could have a negative effect on the patients' adherence to ART, which is a key factor in controlling the disease9.
Different studies have shown that aging is capable of altering virtually all pharmacokinetic processes occurring in the human body, including absorption, first-pass metabolism, bioavailability, distribution, protein binding and hepatic and renal clearance. These alterations play a role in increasing the risk of developing medication-related problems10.
Polypharmacy, which has been defined as the concurrent use of six or more medications11, has become a significant public health problem, particularly in elderly care. Maher et al.12 showed that polypharmacy is associated to negative clinical results and the development of adverse reactions, occurrence of drug-to-drug interactions, use of inappropriate drugs, appearance of malnutrition, worsening of function, falls, fractures and hospital admissions13-15. The Consensus Paper on Old Age and HIV Infection sets at age 50 the cut off point for defining old age in this population. Indeed, there is evidence that HIV causes premature ageing of the immune system and the first signs of impairment of the immune response become apparent at that age. Moreover, the Consensus Paper defines HIV patients aged 65 or over as very elderly11.
Although a few polypharmacy studies on PLWH (people living with HIV) have been performed in Spain16, no representative data are as yet available on elderly persons living with HIV.
The main goal of this study is to determine the prevalence of polypharmacy in elderly PLWH subjected to ART and pharmacotherapeutic monitoring in outpatient pharmacy clinics in Spanish hospitals. Specifically we set about determining the prevalence of comorbidities; describing the different types of ART administered; defining the most commonly used concomitant medication; calculating the prevalence of polypharmacy and major polypharmacy; analyzing the adherence associated to polypharmacy; studying pharmacotherapeutic complexities; and identifying the prevalence of drug-to-drug interactions in the population under study.
Methods
This paper consists in a sub-analysis of the POINT project, whose main purpose was to determine the prevalence of polypharmacy in PLWH receiving ART and subjected to pharmacotherapeutic monitoring at outpatient pharmacy clinics in Spanish hospitals. It is a multi-center cross-sectional observational study that included PLWH aged 65 years and over who were on active ART and were dispensed their antiretroviral medication by the pharmacy department of the participating hospitals on the day on which the cross-sectional sample was drawn (February 2017). The Spanish Drug Agency classified the POINT study as a “post-authorization study using designs other than prospective follow-up”. POINT was authorized by the Ethics Committee of the Sevilla Sur Health District. Its full title was Prevalencia, factores asociados y complejidad farmacoterapéutica de la polifarmacia en pacientes VIH en España (Prevalence, prevalence-related factors and pharmacotherapeutic prevalence of polypharmacy in HIV patients in Spain).
Exclusion criteria were as follows: patients admitted on the day the study was carried out, patients included in clinical trials and those failing to sign the informed consent form.
The demographic variables analyzed were sex and age. Clinical variables included route of acquisition of the HIV infection, viral load and CD4 count, and comorbidities (liver disease (HBV, HCV), central nervous system disease, cardiovascular disease or hypertension, and chronic pulmonary disease (COPD or asthma)) at the time of the study. Comorbidity patterns were classified into cardio-metabolic, pycho-geriatric, mechanico-thyroid or combined, which required for at least two conditions to be present. Other variables analyzed included pharmacotherapeutic variables, type of ART administered (triple-therapy, dual-therapy or other combinations), types of STR (single-tablet-regimen) treatment, and concomitant medication prescribed (obtained from the patients' clinical record and clinical interview). Polypharmacy was defined as the simultaneous prescription of six active ingredients, including those used for ART. Major polypharmacy was defined as the concomitant use of 11 active ingredients or more11. Patients were classified according to their polypharmacy pattern: cardiovascular, depressive-anxious, pulmonary disease, or combined. To fall into one of those categories, patients had to have been prescribed at least three drugs belonging to the same category.
Adherence to ART and to concomitant medication was measured using the dispensation records of the last six months. The SMAQ and Morisky-Green questionnaires were also used, the former for ART and the latter for the concomitant medication. Patients were considered adherent if the possession rate for the relevant medication was over 95% for ART and over 90% for the concomitant medication. The respective questionnaire had to yield a positive result.
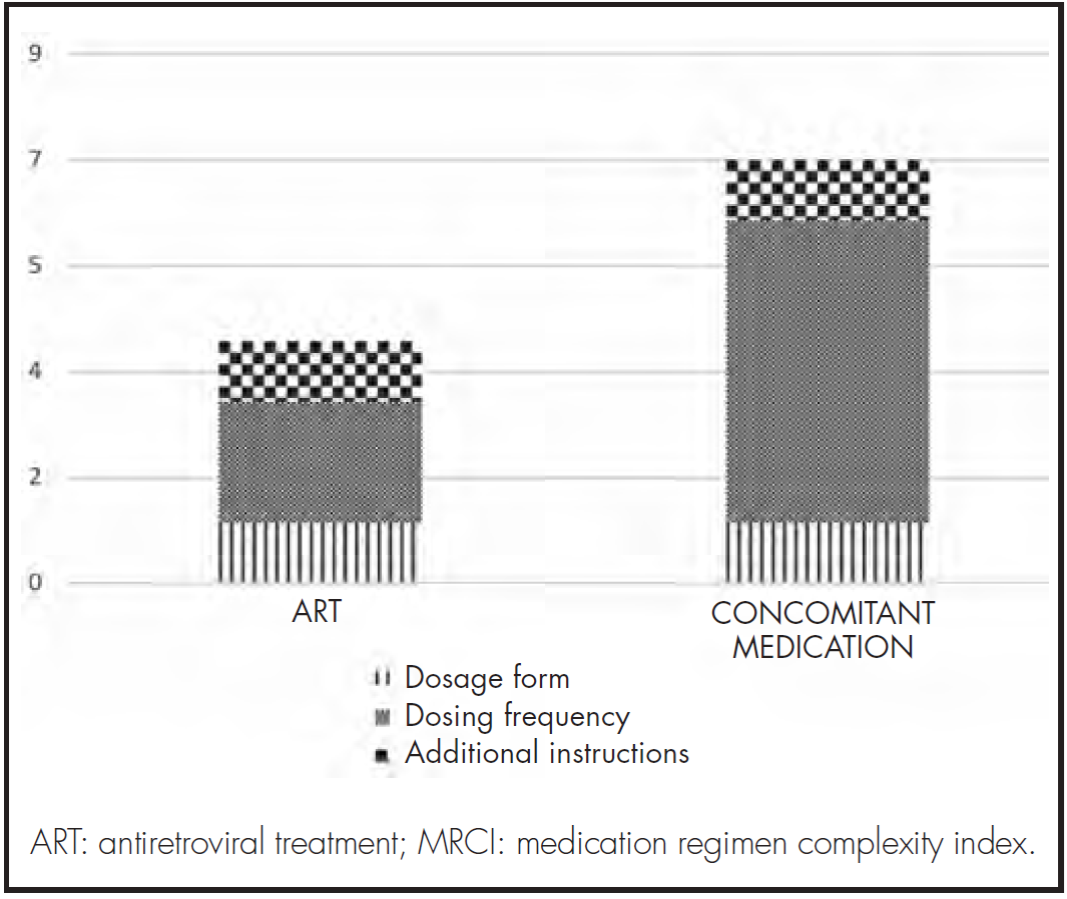
Pharmacotherapeutic complexity was calculated using the MRCI (Medication Regimen Complexity index) index, proposed by George et al.17.
This index allows calculation of medication complexity, considering the following three criteria: dosage form, frequency of dosing and additional administration instructions.
The overall complexity of the medication prescribed was recorded during the data collection process and during the interview leading to the study. In addition, a quantitative and qualitative analysis was made of the complexity of the different subsections making up the MRCI index.
Drug-to-drug interactions, including contraindicated interactions, were evaluated using the Liverpool HIV Pharmacology Group website (www.hiv-druginteractions.com) and the Lexicomp database.
Statistical analysis
At first, a statistical refinement of the data was performed. The resulting data were then individually processed. Quantitative variables were expressed either as means (standard deviation), or through medians and percentiles (P25 and P75) in the case of asymmetrical distributions. Qualitative variables were expressed by frequencies and percentages.
Data were analyzed using the IBM SPSS 20.0 software package for Windows.
Results
Eighty-one hospitals from all 17 Spanish regions participated in the study. In total, 74 PLWH were recruited (86.5% were male), of whom 71.6% were on polypharmacy and 25.6% on major polypharmacy. The median of concomitant drugs prescribed stood at 5.0 (interquartile range (IQR) 2-7). Table 1 shows the socio-demographic and clinical characteristics of patients in the study.
Table 1. Demographic and clinical characteristics of persons living with HIV in the study

CNS: central nervous system; COPD: chronic obstructive pulmonary disease; CV: cardiovascular; HT: hypertension; IQR: interquartile range.
Fifty PLWH had a comorbidity pattern; in 58.0% it was cardio-metabolic, in 12.0% psycho-geriatric, in 6.0% mechanico-thyroid, and in 24.0% combined.
As regards ART regimens, the most frequently prescribed combinations were: two nucleoside/nucleotide-analog reverse transcriptase inhibitors (NRTi's) plus an integrase strand transfer inhibitor (INSTI) (39.2%), and one non-nucleoside analog reverse transcriptase inhibitor (NNRTI) (35.1%). The most frequently used NRTIs, NNRTIs, protease inhibitors (PIs) and INSTIs were abacavir/lamivudine (75.4%), rilpivirine (46.9%), darunavir (92.9%) and dolutegravir (65.8%) respectively. A total of 81.1% of the population received triple therapy, 10.8% received dual-therapy and 4.1% monotherapy. Only 4.1% of the population received other combinations. STR was administered to 48.6% of patients.
Table 2 shows the ART regimens administered under the study.
A total of 56.8% of PLWH were on antihypertensive and cardiovascular medications (C1 to C9 on the ATC classification system); 50.0% were on lipid-lowering agents; 33.8% on antiulcer drugs; 32.4% on psychoactive agents (anxiolytics and antidepressants (N05, N06)); 29.7% on anti-diabetic medication (A10); 10.8% on COPD drugs (R03); 5.4% on antiepileptic agents; and 2.7% on Parkinson's disease medication.
Table 2. Antiretroviral treatment

3TC: lamivudine; ABC: abacavir; COB: cobicistat; DRV: darunavir; DTG: dolutegravir; EFV: efavirenz; ELV: elvitegravir; FTC: emtricitabine; NVP: nevirapine; RAL: raltegravir; RPV: rilpivirine; RTV: ritonavir; TAF: tenofovir alafenamide; TDF: tenofovir disoproxil fumarate.
PLWH were classified according to their polypharmacy pattern, which was cardiovascular in 25.7% of patients, depressive-anxious in 5.4%; chronic respiratory in 5.4% and combined in 2.7%. No prevailing pattern was found in 60.8% of subjects.
The percentage of PLWH that adhered to their ART was 85.1% when calculated on the basis of dispensation records, and 68.5% when the SMAQ questionnaire was used for the calculation. The percentage of PLWH who adhered to their concomitant medication was 62.8% when calculated based on dispensation records, and 65.6% when the Morisky-Green questionnaire was used for the calculation.
The median medication complexity score for the entire regimen (ART + concomitant medication) was 13.0 (IQR 8.0-17.6), with the highest levels of complexity being associated with the dose frequency of the concomitant treatment (Figure 1).
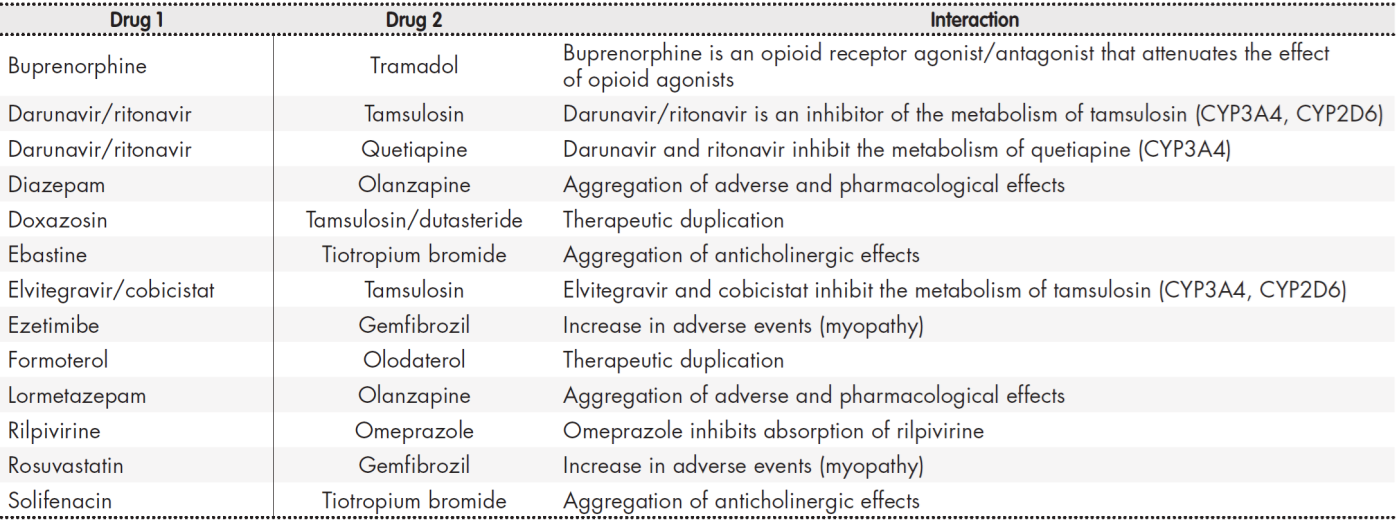
As regards drug-to-drug interactions, 55.4% of PLWH developed at least one potential interaction, and 12.2% at least one contraindicated interaction. The total number of interactions identified was 110 (97 potential interactions and 13 contraindicated interactions, see Table 3), with a mean of 2.6 (2.4) interactions per patient. Pharmacokinetic interactions occurred more frequently (71.8%) than pharmacodynamic ones. Interactions resulted mainly from changes in the metabolism of the drugs (63.3%) and from impaired absorption of the drugs into the body (26.6%). Interactions between the drugs in the ART regimen and the drugs taken concomitantly by the patients (76.6%) were more common than those between concomitant medications.
The NNRTI agent was the antiretroviral agent most commonly involved in drug-to-drug interactions (40.7%), followed by the PI agent (29.6%). The concomitant drugs most usually involved in interactions were statins, oral antidiabetics, urological agents and mineral supplements, with rates of 22.7%, 9.1%, 7.3% and 6.4%, respectively.
Discussion
This cross-sectional study revealed a very high prevalence of polypharmacy and pharmacotherapeutic complexity in PLWH older than 65 years receiving ART in Spain.
The most common comorbidity pattern for the studied population was the cardio-metabolic one, which coincides with the findings of the GEPPO cohort, a large Italian geriatric cohort where dyslipidemia, hypertension and cardiovascular disease are the most frequently occurring comorbidities18. As a result, the predominant polypharmacy pattern was the cardiovascular one, in line with the findings published in previous studies7.
Most patients in the studied cohort were on triple therapy ART and 48.6% were receiving STR. This diverges from the findings by Guaraldi19 where STR regimens were typically administered to younger patients, and LDR (less drug regimen) monotherapy and dual combination therapy were administered to older patients. In contrast to Guaraldi et al., where the most common STR regimens were efavirenz/emtricitabine/tenofovir and rilpivirine/emtricitabine/tenofovir, in our cohort most patients received abacavir/ lamivudine/dolutegravir. This difference could be due to the fact that Guaraldi et al. were unable to avail themselves of the latter combination. The low prevalence of LDR in our population is remarkable, considering that the use of monotherapy and dual combination therapy is one of the preferred strategies for elderly patients to decrease polypharmacy, potential drug-todrug interactions and their consequences20.
The polypharmacy and major polypharmacy rates (71.6% and 25.7%, respectively) observed in the PLWH in our study are somewhat higher than those in the Italian21, Canadian22 and English23 cohorts. These differences may be due to the definition of polypharmacy used. That said, prevalence was high in all the studies mentioned.
Polypharmacy is associated with poorer adherence to ART9. In the population studied, adherence to ART was 85.1% when measured based on the dispensation record, and 68.5% when measured against the SMAQ questionnaire. This disparity is due to the fact that each method has its limitations, each of them yielding a different adherence rate. Adherence to concomitant medication, for its part, was lower than for ART. With the above-mentioned ART adherence rates, viral load was undetectable for 90.9% of patients. This raises questions as to the level of adherence needed to reach viral suppression, which could be drug-dependent and lower than what is generally believed, as suggested by Byrd et al.24.
Higher treatment complexity is associated to a higher likelihood of non-adherence and a higher admission risk; it also often increases after hospital admission25-27. For that reason, reducing complexity would seem to entail significant benefits for the patient. It would appear that, in the subjects in our cohort, a reduction of the MRCI could be achieved by enhancing the dosage of the concomitant medication, as this was the element that contributed the most to the complexity index. Moreover, an MRCI score of 11.25 has recently been found to be the threshold for predicting polypharmacy in PLWH28. We will henceforth have to bear in mind that threshold when identifying our patients and trying to improve their medication regimen.
As regards drug-to-drug interactions, polypharmacy has been shown to be associated to a higher probability of developing an interaction29. This was precisely the case with our population, where 12.2% of PLWH exhibited at least one contraindicated interaction, almost twice as many as in the study by Bastida et al.29 which despite being single-center shows similar results to Greene et al.30.
The main limitation of this study was its cross-sectional design, well suited to quantify polypharmacy but not to identify predictive factors. The size of the sample was small given the characteristics of the study. There was also a limitation related to the methods used to measure adherence which, in spite of their compound nature (questionnaire + dispensations record), are rather inaccurate. The main strengths of the study include its multi-center nature and the fact that it included patients from all 17 Spanish regions.
Future studies should focus on finding ways of reducing polypharmacy and pharmacotherapeutic complexity in elderly PLWH. A set of or bin or bing guidelines for non-ART therapy in HIV patients has been recently published31. This document could be used as a basis for developing specific programs targeted to elderly patients with HIV.
In summary, this study shows how elderly PLWH are characterized by high levels of polypharmacy and pharmacotherapeutic complexity, as well as or adherence to concomitant medications, and a high risk of experiencing drug-to-drug interactions. These results should facilitate the development of pharmacotherapeutic intervention and optimization strategies for these kinds of patients. (Table 4, Table 5, Table 6)
Contribution to the scientific literature
The chief practical contribution of this study is that it provides data on the prevalence of polypharmacy, its associated factors, and pharmacotherapeutic complexity in elderly persons living with HIV in Spain. The data provided herein can be used as a basis for the development of intervention and pharmacotherapeutic optimization strategies for patients on polypharmacy requiring intensive monitoring.











 text in
text in