Introduction
The immune system functions by using checkpoints that modulate T-lymphocyte activity and that can be activated by tumor cells to evade the immune response1. A new anticancer strategy employs immune check-point inhibitors (ICI), which reactivate the antitumor response mediated by T lymphocytes, thus releasing the immune checkpoint blockade in the tumor microenvironment1,2. Nivolumab is an ICI that binds to PD-1, blocking its interaction with PD-L1 and PD-L21.
Desensitization protocols (DP) are strategies to allow the reintroduction of medications that induce hypersensitivity reactions in highly sensitized patients who need first-line therapies. These protocols involve the gradual administration of small amounts of the medication up to its total therapeutic dose. To increase the safety and tolerability of desensitization, patients are premedicated with different drugs that include systemic corticosteroids (SCS) 3. In patients treated with ICIs, the administration of SCSs could adversely affect the therapeutic response due to their immunosuppressive effect. Therefore, it seems justified to avoid the use of SCSs in these patients2.
We describe a case study of a patient who experienced a hypersensitivity reaction to nivolumab. After she underwent a DP without corticosteroids, she safely received 20 doses of the drug.
Case description
A 57-year-old woman, without known drug allergies, but with a medical history of high blood pressure, was diagnosed in April 2015 with stage IV clear-cell renal cancer, with bone and left adrenal gland metastases. She was started on pazopanib 800 mg daily, with stable disease until April 2017, when pulmonary progression was observed. She was started on cabozantinib 60 mg daily, with partial response. In February 2018, after new progression, she was started on nivolumab 3 mg/kg (249 mg) every two weeks. Ten minutes after starting the third infusion cycle, the patient began to exhibit erythema, bronchospasm, and dyspnea with an oxygen saturation of 89% and steady blood pressure. Infusion was stopped immediately, and hydrocortisone 100 mg and dexchlorpheniramine 5 mg were administered, with rapid improvement of symptoms. The Allergy Department performed skin tests (prick test and intradermo-reaction) with negative results.
Two weeks after the adverse event, the patient attended for a new cycle of nivolumab, using a PD stepped up speed. Premedication comprised orally administered cetirizine 20 mg, ranitidine 300 mg, and prednisone 60 mg the night before the cycle. Before starting infusion, the patient received intravenous dexamethasone 20 mg, ondansetron 8 mg, dexchlorpheniramine 5 mg, and ranitidine 50 mg. The Pharmacy Department compounded a single bag of nivolumab 249 mg diluted in 0.9% sodium chloride 249 mL. Based on indications provided by the Allergy Department, the rate of perfusion followed a rapid 15-step scheme subject to patient tolerance until completion of the total drug infusion (Table 1). This perfusion scheme, and an additional one 14 days later, were completed without adverse events.
Table 1. Steps of the desensitization protocol. Single bag of nivolumab 1 mg/ml, nivolumab 249 mg in physiological saline 249 mL*
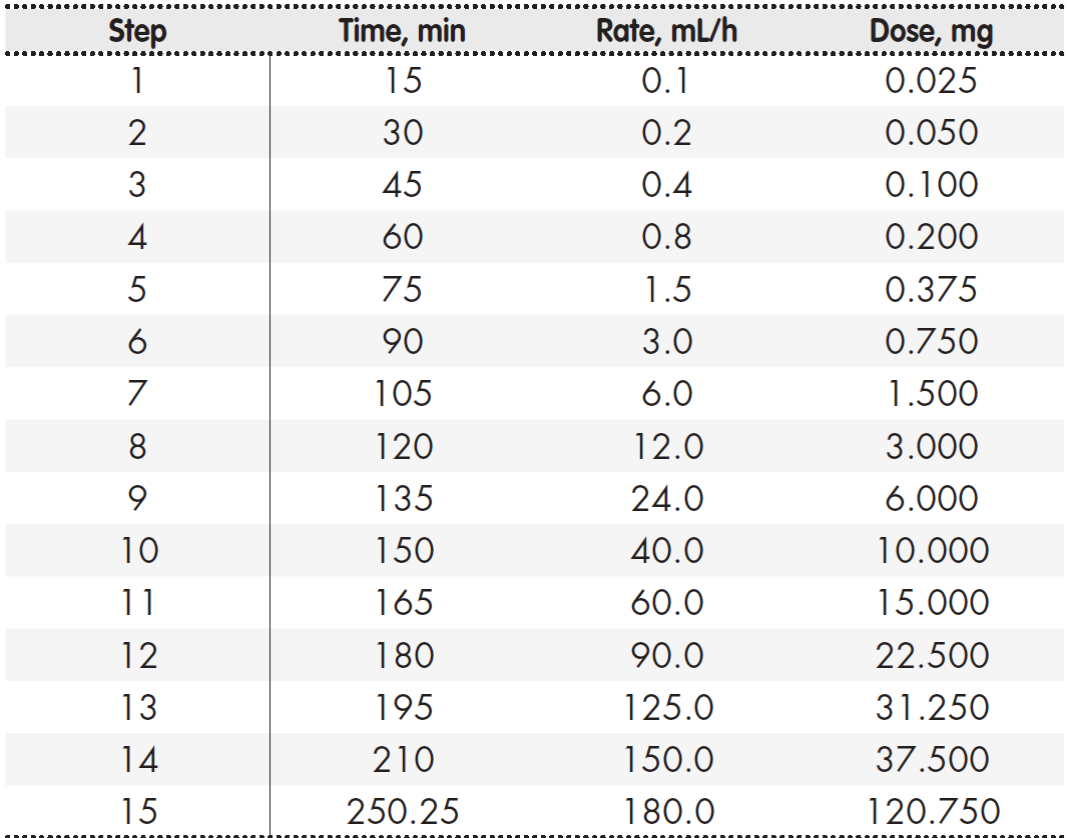
*Total dose administered: nivolumab 249 mg.
In April 2018, in order to avoid possible unwanted interactions between the use of corticosteroids and immunotherapy, the Pharmacy Department suggested the possibility of developing a DP without corticosteroids. This DP was subsequently designed by the Allergy Department. Under the new protocol, premedication was given 24 hours and 1 hour before the initiation of the DP. Premedication comprised acetylsalicylic acid 300 mg, ranitidine 300 mg, montelukast 10 mg, and cetirizine 20 mg. Prior to this cycle, treatment was discontinued due to suspicion of clinical progression. After radiological reassessment, brain metastases and a partial response at the extracerebral level were observed, and so DP to nivolumab without planned corticosteroids was resumed.
At the time of writing (May 2019), and after 20 administrations without any adverse effects, the patient remains in treatment with stable disease and with a good general condition.
Discussion
Systemic corticosteroids induce apoptosis of effector T cells, altering memory T cells and regulatory T cells. One hypothesis suggests that, due to their mechanism of action, the immunosuppressive effect of SCSs could weaken the effectiveness of ICIs2,4. However, there are limited and contradictory data on the effect of the concomitant use of high doses of corticosteroids on the course of immunotherapy treatment.
A retrospective review of 210 patients with non-small-cell lung carcinoma (NSCLC) found that the group that was exposed to SCSs during the first nivolumab cycle had a lower response rate and shorter overall survival (OS) than those who did not2. A systematic review of SCS and ICI interactions did not come to a clear conclusion on interference between SCSs (type and dose) and immunotherapy, suggesting that the concomitant administration of SCSs and ICIs may not necessarily lead to worse clinical outcomes5. Another retrospective study in PD-(L)1-naive patients with advanced NSCLC found that the initiation of SCSs during treatment with ICIs does not affect their efficacy, whereas the use of previously established SCSs remained associated with a worse response rate, decreased progression-free survival, and decreased OS. The prudent use of SCSs when initiating ICIs was recommended4.
We consider that it would be useful to design a DP that avoids the use of SCSs in premedication and uses prostaglandin blockers and/or leukotriene inhibitors given that the literature has provided examples of their safety and efficacy6,7. Our DP was simpler and shorter than traditional schemes because of the use of a single bag, which also guaranteed stability and minimized handling errors8. After a literature search of DP to nivolumab, we found a single case of a 14-step protocol using the classic three-bag scheme, which included SCSs in premedication9,10. However, we found no studies that addressed DP to immunotherapy without SCSs.
Our experience has demonstrated the safety of using a DP to nivolumab without SCSs, which did not compromise the effectiveness of treatment.











 text in
text in 


