Introduction
Over the last few decades, hospital pharmacy departments (HPDs), as well as hospital pharmacy as a healthcare specialty, have undergone significant changes and enjoyed considerable professional growth. These developments, which have brought about changes in the legislation, were prompted by an increasing need for specialized pharmaceutical care and were possible largely thanks to the dedication and hard work of hospital pharmacists. Some of the changes included the creation of specialized outpatient units1,2, the incorporation of pharmacists to the clinical work of inpatient and emergency wards3,4, the addition of a fourth year to the hospital pharmacy residency program5, the appointment of hospital pharmacists to elderly care centers6, and the adoption of innovative technologies to enhance medication logistics7,8, among others.
In the last few years, several surveys have been conducted, most of them organized by one of the working groups of the Spanish Society of Hospital Pharmacists (SEFH), to assess the state of affairs in the different domains of pharmaceutical practice. One such survey is the one periodically administered by SEFH's Grupo 20209, which consists of a series of specific questionnaires on the activities carried out by the different working groups under SEFH, such as GEDEFO, REDFASTER, MAPEX, etc.10-12. Mention should also be made of the medication self-assessment questionnaire prepared by ISMP-Spain in collaboration with SEFH13 and the Europe-wide surveys conducted in association with the European Association Hospital Pharmacy (EAHP)14.
Despite all the work done, for a long time there was no document that took stock of the overall situation of HPDs in Spain. Eventually, in 2014 a survey was conducted that resulted in the publication of the Informe sobre la situación de los Servicios de Farmacia Hospitalaria en España: Infraestructuras, recursos y actividad15 (Report on the situation of hospital pharmacy departments in Spain: infrastructures, resources and activity), known as the “White Paper” of Hospital Pharmacy. The idea was to make the government, society and HPDs themselves aware of the characteristics and dimensions of hospital pharmacy from the healthcare, technological, educational and investigational points of view.
Four years later, in 2019, SEFH put together a similar survey with a view to preparing a second white paper of hospital pharmacy that would provide an update on the situation of hospital pharmacists and HPDs, analyzing their evolution in the past four years.
The purpose of this article is to introduce the results of SEFH's 2019 national survey on the situation of Spanish HPDs, specifically in terms of general characteristics, human resources, materials, and information systems.
Methods
In 2014, SEFH's Board of Trustees designed a survey containing 78 questions grouped into 8 dimensions. There was an additional group of 7 questions relative to the activities of HPDs in 2012 and 2013.
In 2019, a second, similarly designed survey was planned. SEFH's Board of Trustees set about preparing the new survey, which came to contain 77 questions also grouped into 8 dimensions. The updated survey also contained a section of questions on the activities of HPDs in 2017 and 2018, drawing on the HPD product and payment catalog published by SEFH16. The 8 dimensions of the questionnaire were as follows: 1) characteristics of the hospital and its HPD; 2) services offered; 3) human resources;
4) material resources, 5) information systems, 6) quality and accreditation;
7) research, 8) training.
The questionnaire was administered online and participation was voluntary. It was sent by SEFH to the heads of the different HPDs in Spain, as per the information recorded in SEFH's member directory. SEFH contacted the HPD heads by letter up to three times in February 2019 to inform them about the survey. The information was also disseminated across the different Autonomous Regions through SEFH's regional representatives. Answers were processes between March and September 2019.
Each hospital was assigned its own identifying number. Results were analyzed as weighted mean differences, taking into consideration the definition of the universe obtained from the SEFH member directory, together with a classification of hospitals based on the 2019 National Hospital Catalog. In order to infer the results at a national level, the weighting was based on two classification variables: whether the hospital was public or private and the number of beds available in each of them, according to the National Hospital Catalog (five categories were established). Responses from hospitals underrepresented in the sample were assigned a weighting proportional to their representativeness in the universe, with a 3% error margin. Weightings also took into consideration the response rate obtained for each question, as we anticipated that the weighting of the sample would not be uniform or proportional.
The information was collected and analyzed using the OBM Statistics SPSS® software (version 22.0). A descriptive analysis was conducted of all the answers. Qualitative variables were expressed as frequency distributions and quantitative variables as mean and median values and dispersion measures.
Results
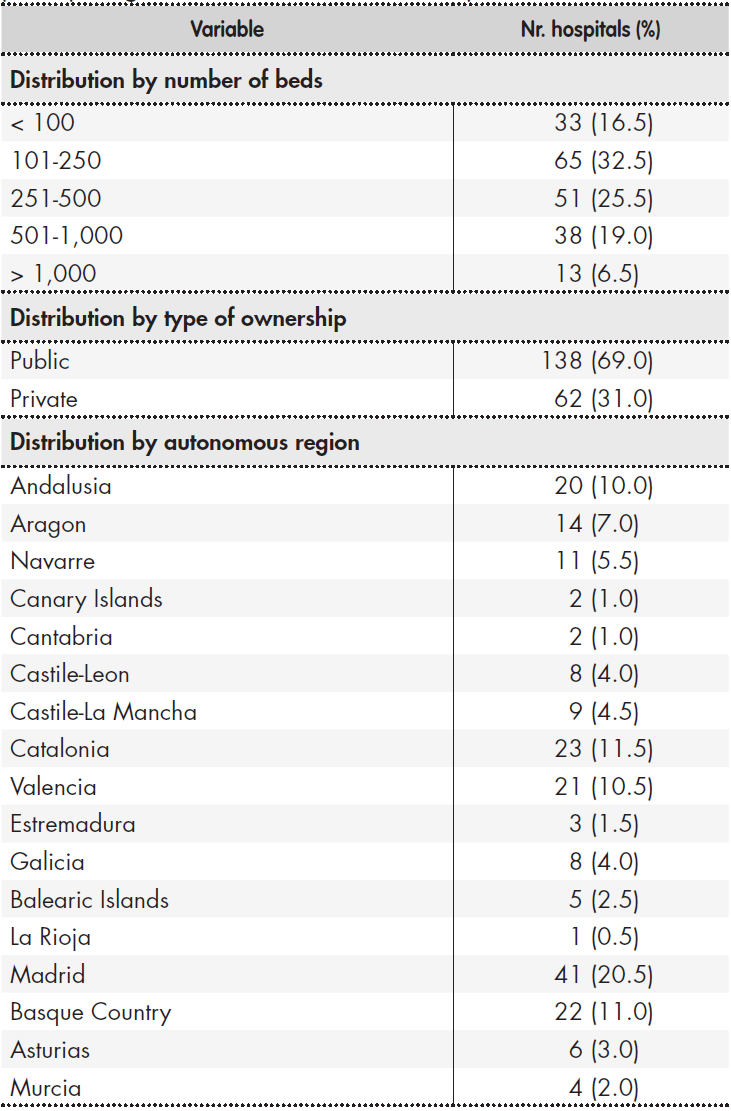
A total of 368 HPDs were invited to participate in the survey and 200 responses (54.3%) were received. Table 1 shows the distribution of participating hospitals classified by type of ownership, number of beds and location. Sixty-nine percent of participating hospitals were publicly owned, and most were in the 101-200 bed range. Responses were obtained from hospitals across all Spanish autonomous regions, except for the cities of Ceuta and Melilla. Madrid was the region with most respondents, with 20.5% of the total, followed by Catalonia (11.5%), the Basque Country and Valencia.
Table 1. Size, type of ownership and location of hospitals participating in SEFH's 2019 National Survey
Hospital and HPD characteristics
The results regarding the general characteristics of HPDs are shown int Table 2. As regards their operating hours, 9% of HPDs remained open round the clock whereas 39.5% were open either only in the morning or until 5 pm. As far as the availability of continued pharmacological services is concerned, 57.5% of HPDs did not offer this kind of service on weekdays. A total of 39.2% of HPDs had some sort of continued care module that was available for less than 24 hours a day or on a located module.
Table 2. General characteristics of HPDs, according to the criteria in the National SEFH 2019 Survey

FIR: farmacéutico interno residente (pharmacy resident); HPDs: hospital pharmacy departments; SD: standard deviation; Wd/We weekdays/weekends.
One in every three HPDs was accredited to offer a pharmacy residency program (FIR). The percentage of such hospitals with a (resident-staffed) continuing care module in place was 17.4% on weekdays and 20.25% at weekends.
Dispensation to outpatients of medication in the afternoons was available in 41.9% of hospitals, with privately-owned hospitals accounting for the majority (56.7%). A total of 61.5% of the larger hospitals offered pharmacist care and drug dispensation both in the morning and the afternoon.
The most common accreditation among certified hospitals was ISO 9000, held by three out of four certified centers, followed by the EFQM Excellence Model, obtained by 28.15%, the standards in the ISO 14000 series (18.1%) and the Joint Commission International accreditation (10.9%). A total of 12.7% privately owned hospitals operated under the latter quality model.
HPDs were responsible for the management of medical products in 70.5% of privately-owned hospitals, with medical gases and radiopharmaceuticals being managed by HPDs in 46.2% of the total hospital sample and in 53.8% of larger hospitals.
Human resources
The mean number of specialist hospital pharmacists per HPD was 5.34 (SD: 6.22). This figure varied across departments (mean: 3, IQR: 1-8), as a function of both type of hospital ownership and hospital size. Twenty percent of the staff carried out roles of responsibility (Table 3). Overall, 34.8% of pharmacists did not have a permanent contract; the figure was 39.6% in publicly owned hospitals. The most common age group was 41-60 years (54.5% of total).
Table 3. Specialist pharmacists working in HPDs
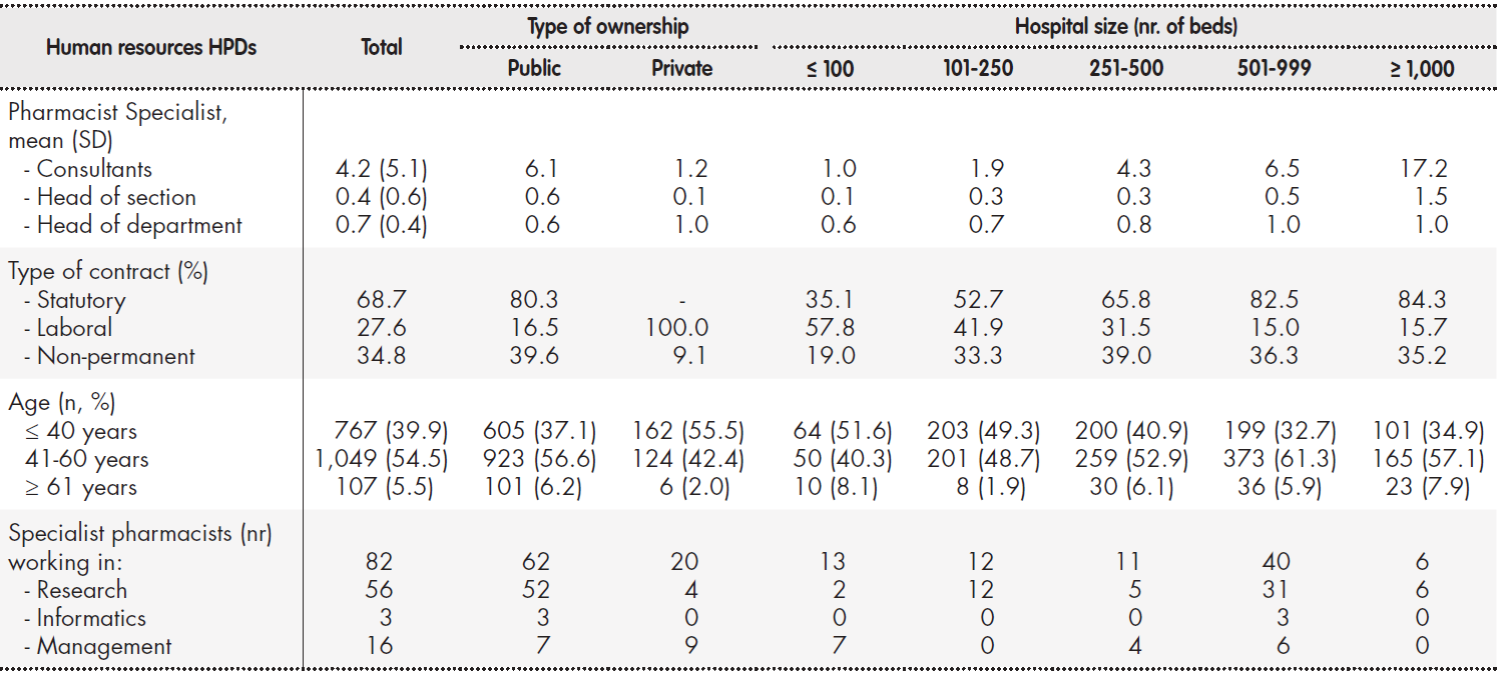
HPD: hospital pharmacy department; SD: standard deviation
Regarding the non-pharmacist staff working in HPDs, the most commonly represented profiles were nursing care assistant, with a mean of 4.06 per HPD (SD:7.07; median: 1; IQR: 0-4); and pharmacy technician, with a mean of 3.95 per HPD (SD: 6.14; median: 2; IQR: 0-5). Figure 1 provides an overview of the number of non-pharmacist staff working in HPDs, classified by type of hospital ownership.
Concerning the number of pharmacists devoting at least half of their working day to clinical work, the mean figure was 2.53 (SD: 4.91) per HPD. In hospitals with over 250 beds, departments where these pharmacists spent at least half of their working day included oncology, hematology and infectious diseases, in that order. Table 4 shows the hospital units with greater pharmacist presence and the mean number of pharmacists in each HPD.
Table 4. Number of specialist pharmacists spending at least half their working day in a clinical unit
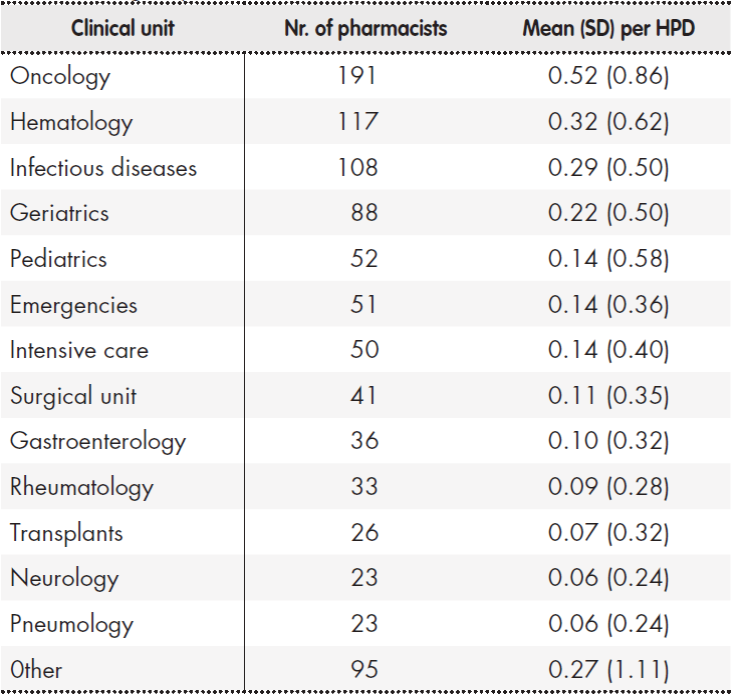
HPD: hospital pharmacy department; SD: standard deviation.
Material resources
Storage, distribution and dispensing systems
As regards the availability of automatic medication storage and dispensing carousel systems, HPDs reported having such systems in both their horizontal and vertical modalities. The mean number of those systems available in each HPD was 0.3 (SD: 0.7) and 0.9 (SD: 1.4), respectively. Overall, 16.1% of hospital beds were covered by an automated dispensation system, that figure jumping to 26 and 33.5% in hospitals of over 500 and over 1,000 beds, respectively. One in every 10 HPDs boasted automated systems for dispensing medication to outpatients (Table 5).
Table 5. Material resources associated to the compounding and distribution of medications in Spanish HPDs

BSC: biosafety cabinet; HPDs: hospital pharmacy departments; IVM-PN: intravenous mixtures and parenteral nutrition; OPs: outpatients; SD: standard deviation.
Systems associated to the preparation, compounding and packaging of medications
The mean number of clean rooms in HPDs was 1.29 (SD: 1.49, median: 1 (IQR: 0-2); this figure went up to 3 in larger hospitals (Table 5). Robots for compounding chemotherapy or other intravenous drugs were available in 3 and el 4% of HPDs, respectively.
Table 6. Systems associated to medication traceability and safety in HPDs

HPDs: hospital pharmacy departments.
Medications traceability and safety systems
Table 6 shows the degree of implementation of different technologies associated with pharmacological traceability.
One in every 3 HPDs used a barcode system, either linear or bidimensional, for receiving and dispensing medications. This figure rose to 4-6 in every 10 HPDs in larger hospitals. A total of 2.4% of hospitals employed a radiofrequency system.
With respect to traceability systems for chemotherapy compounding, one in every 4 HPDs had one such system; this figure increased to 46.2% in larger hospitals. Traceability systems for preparing parenteral nutrition formulations and other intravenous admixtures were less common: only 14.8% of HPDs had systems of that nature in place. Smart infusion pumps were in use in 21.4% of hospitals surveyed.
Information systems
A total of 4.9% of hospitals did not use electronic medical records (EMRs). This figure rose to 10.7% and 15.4% in hospitals with more than 500 and 1,000 beds respectively. Use of a single electronic medical record, shared with primary care, was reported by 27.7% of HPDs overall and by 30.8% of HPDs in the larger hospitals.
Electronic prescriptions were implemented in 98.8% of hospitals for hospitalized patients, and in 49.7% and 62% for outpatients and inpatients, respectively. These figures rose to 81.8% and 100% in hospitals with over 1,000 beds. When asked about the percentage of patients who recei-ved such electronic prescriptions, 92.1% of respondents stated that they were delivered to over 75% of admitted patients, 85.1% of outpatients, and 78.3% of inpatients.
The administration of medication was electronically recorded in 74.1% of hospitalized patients and 44.4% of day inpatients. Of the hospitals that had this system, it was applied in over 75% of the hospitalized patients and day inpatients, in 49,9% and 41.9% of the hospitals, respectively.
When asked whether their HPD had a remote consultation system or telepharmacy in place, 86.4% of respondents stated they had no such system. Of the 13.6% that had a remote patient care service, 65.4% said that delivery of the medication associated with those remote consultations took place in the patient's health center, while 27.9% reported that the medication was delivered at the patients' home.
Discussion
In 2014, SEFH conducted the first survey addressed to HPD directors in Spanish hospitals. The survey provided insight into the situation of HPDs at a national level15. A second survey was conducted in 2019, which showed the development HPDs had undergone over the intervening period.
The 2019 SEFH survey was completed by over 50% of the HPDs invited, which is testament to the high level of engagement of HFDs in Spain. In fact, surveys conducted in other countries, such as those administered every year by the American Society of Hospital Pharmacists (ASHP)17-20and those recently conducted in Europe/21,22) show lower response rates (response rates for the ASHP surveys have typically ranged between 10.8% and 29.8%). The SEFH survey received more responses from public than from private hospitals, although it must be noted that the response rate of private centers showed an increase with respect to the rate obtained in 2014.
Proper performance of their functions makes it necessary for HPDs to count on a certain level of staffing, infrastructure, equipment and technology. The survey showed that less than 10% of HPDs were open round the clock and over half of them lacked a continuing care module or even a pharmaceutical emergency service, which was in line with the figures recorded in 2014.
Almost all hospitals in the sample provided care of and dispensation to outpatients. This contrasts with the situation in the United States, where such services are not offered by over half of HPDs. In fact, only 18% of HPDs in the United States are specifically accredited to perform those activities17. With regard to the availability of such services, all HPDs in the sample delivered them at least five days a week, and more than 4 out of 10 did so both in the morning and the afternoon, with slight increases as compared with the figures recorded in 2014.
A generalized interest in continuous improvement was observed across the sample, reflected in the high number of HPDs that had obtained accreditation to a quality standard. The standards most commonly sought were those in the ISO 9000 series, although a twofold increase was observed with respect to 2014 in the number of HCPs accredited to the Joint Commission International, which is known for its stringent patient and medication safety standards23.
Apart from medications themselves, the role of hospital pharmacists also extends to medical devices, including the ones covered by Royal Decree 1/201524, which stipulates that pharmacists should also participate in and coordinate the management of such products. However, although a certain increase was observed in the number of HPDs involved in the procurement and storage of medical products (as compared with 2014), the percentages are still low, particularly in public hospitals. This is at odds with the fact that, according to the Spanish Agency for Medicines and Medical Devices (AEMPS), medical devices are included in the so-called pharmaceutical channel and the risk management of such devices is placed in the hands of HPDs. On the other hand, HPDs tend to be responsible for the management of medical devices in privately owned hospitals. In the case of medical gases and radiopharmaceuticals the situation is often different, as they are taken care of by HPDs in only 50% of the larger hospitals. It must be mentioned, however, that the participation of HPDs in the management of radiopharmaceuticals is strongly dependent on the availability of a nuclear medicine department in the hospital.
Although in the past it was the number of beds in the hospital that
determined the number of pharmacists in an HPD, nowadays other activity indicators have come into use. The 2012 EAHP survey25 included a comparison of European countries in terms of their ratio of pharmacists per 100 occupied beds. The mean ratio across participating countries was
1.1 (median: 0.9), with significant differences between countries, as ratios ranged from 0.24 in Bosnia Herzegovina to 4.35 in the United Kingdom. Spain ranked sixth, with a ratio of 1.5 pharmacists per 100 beds. The data or the survey analyzed in the present study reveal a slight increase, below 10%, in the number of specialist pharmacists in a HPD with respect to 2014, with the mean currently standing at 5.34 (SD: 6.22), by HPD.
An analysis of the number of pharmacists in hospitals of different sizes reveals that the higher pharmacist/number of beds ratio is observed in hospitals with less than 101 beds (2.7 pharmacists per 100 beds); larger hospitals show ratios between 1 and 2 pharmacists per 100 beds. A comparison with the United States reveals significant differences. In fact, the mean number of full-time pharmacists per 100 occupied beds in 2019 (hospitalized patients) was 19.2 and ranged between 25.9 in hospitals with less than 50 beds to 14.1 in those with more than 600 beds26.
The dearth of specialist pharmacists in Spanish hospitals is also brought out by the results of the latest EAHP Statements Survey, focused on sections 2, 5 and 6 of the European Statements of Hospital Pharmacy22. Seventy-six percent of hospitals that completed that survey reported less than 10 pharmacists, whereas in our study a similar percentage of hospitals reported less than 8 pharmacists, which is below the acceptable threshold established in the EAHP survey22.
With respect to the types of contracts held by hospital pharmacists, one in every three did not have a permanent contract, which is higher than the temporary employment rate of the country's public sector (27.8%) according to the 2019 Economically Active Population Survey27. This confirms the perceptions of precariousness associated to this segment of the labor market.
The mean number of non-pharmacist staff working in HPDs was below 14, most non-pharmacist positions being occupied by nursing assistants. The survey revealed there had been a significant increase in the number of pharmacy technicians and a reduction in the number of nurses as compared with 2014. A comparison with the situation in the United States shows that the number of technicians per 100 occupied beds in that country is also higher than the figure for Spain by HPD, including all non-pharmacist professional categories29.
The incorporation of pharmacists to multidisciplinary care teams and to the different specialist units in the hospital is a key strategic goal for Spanish HPDs and scientific societies28 as it has been shown to significantly contribute to optimizing medication management29. The survey shows that a mean of nearly 2.5 pharmacists per HPD spend at least part of their working day in clinical units, which represents a twofold increase with respect to the situation in 2014. Pharmacists were already doing work in oncology, emergency and infection disease units, and have now become actively involved in the work of geriatric, critical care, transplant, and surgical departments. The survey also shows a slightly increased pharmacist participation in the gastroenterology, neurology, pneumology and rheumatology units. According to Pedersen et al., the ASHP survey shows that oncology departments in United States include at least one pharmacist in their teams, which is in line with the figure observed in Spain. Nevertheless, pharmacists in the US system play a much more active role than those in Spain in intensive care units, surgical and medical areas and, to a lesser extent, in infection disease and emergency units, to mention only those devoted to hospitalized patients17.
One of the main priorities of HPDs in the last decade has been the
automation of the logistic and dispensing process, semi-automatic carousels being one of the key developments in this area. As compared with the results of the 2014 survey, the 2019 questionnaire revealed that technological enhancements had been implemented in both horizontal and vertical storage carousels.
It should also be noted that automated dispensing systems are available for only 16% of hospital beds, which indicates that single-dose dispensing remains the most widespread system used by hospitals for their inpatients. As regards logistic and distribution technologies, the results of the survey are in line with the conclusions of a recent technological evaluation report30, according to which automation of outpatient pharmaceutical services is not economically feasible in hospitals of less than 300 beds, and its implementation in hospitals between 600 and 900 beds for outpatients and of 900 beds for inpatients would depend on the situation of each specific hospital. A solution for larger hospitals would be to introduce the robotization systems for outpatients and automates dispensing for inpatients.
One of the main goals of the 2019 survey was to take stock of the equipment available to compound sterile preparations, an activity included in the service offering of all Spanish hospitals with over 250 beds, in line with the practice of other countries17,22. The results showed that while the availability of clean rooms and laminar flow booths seemed to meet the needs of responding hospitals, the proportion of HPDs using robots to compound cytotoxic medications and nutritional solutions and other intravenous admixtures did not exceed 3 and 4%, respectively. Moreover, despite the interest generated in the last few years, implementation of non-robotized traceability and safety systems for sterile preparations is still around 25% overall. The proportion jumps to 50% in the larger HPDs, particularly in the case of chemotherapy, hazardous medications and, to a lesser extent, other drugs. The situation of smart infusion pumps is not dissimilar, although privately owned hospitals have stepped up their efforts in this respect. The annual ASHP surveys indicate that, in 2018, 19.8 % of US hospitals used some kind of software to manage these activities and 35.7% of hospitals used a barcode scanner to identify each medication during compounding. Other systems, such as image recognition and video surveillance were in use in 19.5% of hospitals, with 4.4% of them using a gravimetric method. In contrast, 56.4% of respondents did not use any technology to assist them in their sterile compounding activities26. On the other hand, only 25% of the US hospitals having that technology use it to compound more than 75% of the doses they prepare. SEFH's survey did not include any question asking about specific types of technology, but it is safe to assume that 75% of hospitals did not have a traceability system in place, as compared with 56.4% in the United States.
The EMR is probably one of the most significant technological advances in healthcare. Access to patients' EMRs by pharmacists, as well as the latter's leadership in the realm of electronic prescriptions and the possibility to record pharmaceutical interventions, have all helped raise the profile of hospital pharmacists and facilitate their inclusion into multidisciplinary care teams31. In 2019, less than 5% of HPDs reported that their hospital did not have an EMR system implemented but hospitals of all sizes had initiatives to set up such a system, in line with their US counter-parts26. Regarding integration of EMR into primary care, an increase with respect to 2014 was observed with one in every three hospitals having completed such integration. Implementation of electronic prescriptions for hospitalized patients experienced a considerable expansion with virtually all hospitals and over 75% of hospitalized patients having access to them. Although the growth of e-prescriptions in the case of outpatients and day inpatients was more limited, larger hospitals had made some headway in that direction.
It should be mentioned that SEFH's 2019 survey showed significant progress in the implementation of the electronic medication administration record (eMAR), which rose from 44.8% of HPDs in 2014 to nearly 70% for hospitalized patients. An increase of over 25% was also observed in the case of ambulatory patients.
Lastly in 2019 remote pharmaceutical care was available in 15% of hospitals, which reflects a keen interest by the pharmacist profession in its development. Moreover, only a few months after the survey was completed, remote care saw a phenomenal surge as a result of the outbreak of the SARS-CoV-2 pandemic27. In 2017, ASHP published its Statement on Telepharmacy, where telepharmacy is given a broader interpretation that in SEFH's survey as it includes not only patient care but also the delivery of remote assistance for different activities related to pharmaceutical practice32. SEFH has since made public its own Position Statement on Telepharmacy, where it adopts a similar approach to that of the ASHP, defining telepharmacy as the remote delivery of pharmaceutical care through information and communication technologies33.
The results of the survey presented in this study are faced with certain limitations, including the fact that the survey was voluntary and rather lengthy and complex. Some of the questions were not easy to understand and, in some cases, respondents had to figure them out by themselves. Furthermore, the size of the sample does allow for hard-and-fast conclusions to be drawn or for improvements to be planned out. Comparisons with the 2014 survey should be taken with caution as the universe of HPDs considered then was different from that in the 2019 survey.
In short, the data obtained from the 2019 SEFH survey suggest that HDPs in Spanish hospitals are understaffed with respect to specialist pharmacists. It must be said however that the number of pharmacists integrated into clinical units experienced a twofold increase as compared with 2014. A greater presence of automation was observed in the logistics of medication dispensing, while there is still significant room for improvement in terms of making the compounding process safer and more traceable. Access to these results may be of great assistance to HPDs, and to SEFH as a whole, in establishing the relevant action plans to address the concerns highlighted by the survey. SEFH is committed to periodically updating this information as part of its efforts to monitor and encourage the development of hospital pharmacy in Spain.
Contribution to the scientific literature
This study provides data about the overall situation of Spanish hospital pharmacy departments in terms of their structure, human resources and technological capabilities. Although some published studies do review the results of questionnaires pertaining specifically to the realm of hospital pharmacy, none of them provides an overview of hospital pharmacy departments, stratifying them based on type of hospital ownership and hospital size. This work should be used as a foundation upon which annual surveys including the different dimensions of our specialty are developed, following the example of the European Association Hospital Pharmacy and the American Society of Hospital Pharmacists. The comparative outcomes extracted from such surveys would allow an in-depth understanding of the situation facing hospital pharmacy department, which would be a first step toward improving the standards of our profession.
List of participating HPDs:
Andalucía: Complejo Hospitalario de Especialidades Juan Ramón Jiménez (Huelva), Complejo Hospitalario Regional Reina Sofía (Córdoba), Hospital Universitario Virgen Macarena (Sevilla), Hospital Comarcal Valle de los Pedroches (Pozoblanco), Hospital Universitario de Puerto Real (Puerto Real), Hospital Universitario de Valme (Sevilla), Agencia Sanitaria Hospital Costa del Sol (Málaga), Agencia Sanitaria Hospital de Poniente (Almería), Hospital Universitario Torrecárdenas (Torrecárdenas), Hospital Dr. Pascual (Málaga), Hospital San Juan de Dios del Aljarafe (Sevilla), Centro Asistencial San Juan de Dios de Málaga, Hospital Mediterráneo Grupo HLA (Almería), Hospital QuirónSalud (Córdoba), Hospital Vithas Xanit Internacional (Benalmádena), Hospital Universitario de Jaén (Jaén), Hospital Punta de Europa (Algeciras), Hospital Santa Ana (Motril), Hospital Cruz Roja Española (Córdoba), Agencia Sanitaria Alto Guadalquivir (Andújar). Aragón: Hospital Maz (Zaragoza), Clínica Montpelier (Zaragoza), Hospital San Juan de Dios (Zaragoza), Centro Neuropsiquiátrico Ntra. Sra. del Carmen (Zaragoza), Hospital Viamed Montecana (Zaragoza), CRP Nuestra Sra. del Pilar (Zaragoza), Hospital Royo Villanova (Zaragoza), Hospital Real de Nuestra Sra. de Gracia (Zaragoza), Hospital Ernest Lluch (Calatayud), Hospital General de la Defensa (Zaragoza), Hospital Universitario Miguel Servet (Zaragoza), Hospital de Jaca (Jaca), Hospital San José (Teruel), Hospital Clínico Universitario Lozano Blesa (Zaragoza). Cantabria: Hospital Comarcal Sierrallana (Torrelavega), Hospital Universitario Marqués de Valdecilla (Santander). Castilla y León: Complejo Asistencial de Ávila (Ávila), Complejo Asistencial de Zamora (Zamora), Hospital Universitario de Salamanca (Salamanca), Complejo Asistencial de Soria (Soria), Clínica Santa Teresa (Ávila), Hospital Comarcal Santiago Apóstol (Miranda de Ebro), Hospital El Bierzo (Ponferrada), Hospital Clínico Universitario de Valladolid (Valladolid). Castilla-La Mancha: Hospital Universitario de Guadalajara (Guadalajara), QuirónSalud (Ciudad Real), Complejo Hospitalario Universitario de Albacete (Albacete), Hospital Nacional de Parapléjicos (Toledo), Hospital Virgen de la Luz (Cuenca), Hospital General de Almansa (Almansa), Hospital General La Mancha Centro (Alcázar de San Juan), Hospital General de Tomelloso (Tomelloso), Hospital General Universitario de Ciudad Real (Ciudad Real). Cataluña: Hospital Universitari Sagrat Cor (Barcelona), Mutual Midat Cyclops (Barcelona), Hospital sociosanitario Mutuam Güell (Barcelona), Hestia Palau (Barcelona), Hospital Clinic Barcelona (Barcelona), Corporacio Salut Maresme i la Selva (Barcelona), Hospital Dos de Maig (Barcelona), Hospital Universitario de la Santa Creu i Sant Pau (Barcelona), Nou Hospital Evangelic (Barcelona), Hospital Universitario Vall d`Hebrón (Barcelona), Hospital del Mar (Barcelona), Parc sanitari Sant Joan de Déu (Sant Boi de Llobregat), Clínica Girona (Girona), Hospital de Sant Celoni (Sant Celoni), Institut Catala d'oncologia (Hospitalet de Llobregat), Badalona Serveis Assistencials (Badalona), Hospital de Terrassa (Terrassa), Hospital Universitari Arnau de Vilanova (Lleida), Hospital Mútua Terrassa (Terrassa), Hospital de Mataró (Mataró), Fundació Hospital Esperit Sant (Santa Coloma de Gramenet), Hospital Sant Joan de Déu (Esplugues de Llobregat), Hospital Universitario de Bellvige (Hospitalet de Llobregat). Comunidad de Madrid: Hospital Universitario de Torrejón (Torrejón de Ardoz), Hospital Universitario Moncloa (Madrid), Hospital Fraternidad-Muprespa (Madrid), Fundación Vianorte Laguna (Madrid), Hospital Universitario La Paz (Madrid), Hospital Universitario La Princesa (Madrid), Hospital Universitario Infanta Sofía (Madrid), Hospital Fuensanta (Madrid), Hospital Universitario Puerta de Hierro (Madrid), Hospital Universitario Severo Ochoa (Leganés), Hospital Universitario de Móstoles (Móstoles), Hospital del Henares (Coslada), Hospital Central de la Defensa (Madrid), Hospital Universitario Infanta Leonor (Madrid), Hospital Universitario Sanitas La Moraleja (Madrid), Hospital Universitario Fundación Jiménez Díaz (Madrid), Hospital Clínico San Carlos (Madrid), Hospital Universitario 12 de octubre (Madrid), Hospital del Tajo (Madrid), Centro Penitenciario Madrid VII (Madrid), Hospital Infantil Universitario Niño Jesús (Madrid), Hospital Virgen de la Poveda (Villa del Prado), Hospital General Universitario Gregorio Marañón (Madrid), Hospital Universitario Príncipe de Asturias (Alcalá de Henares), Hospital Universitario de Fuenlabrada (Fuenlabrada), Hospital de la Zarzuela (Madrid), Hospital Universitario Fundación Alcorcón (Alcorcón), Hospital HM Torrelodones (Torrelodones), Hospital Guadarrama (Guadarrama), Hospital Central de la Cruz Roja (Madrid), Hospital La Fuenfría (Cercedilla), Casa de las Hermanas Hospitalarias del Sagrado Corazón de Jesús (Ciempozuelos), Hospital Universitario Infanta Cristina (Parla), Hospital José Germain (Leganés), Hospital Virgen del Mar (Madrid), Hospital El Escorial (El Escorial), Hospital QuirónSalud San José (Madrid), Hospital Universitario Ramón y Cajal (Madrid), Hospital Universitario del Sureste (Arganda del Rey), Hospital Universitario de Getafe (Getafe). Comunidad Valenciana: Hospital Intermutual de Levante (Valencia), Hospital Universitario de Vinalopó (Elche), Hospital Universitario Torrevieja (Torrevieja), Hospital Clínico Universitario de Valencia (Valencia), Hospital Universitario de Sant Joan (Alicante), Sociosanitario La Florida (Alicante), Hospital de La Magdalena (Castellón de la Plana), Hospital de Sagunto (Sagunto), Hospital Universitario Dr. Peset (Valencia), Hospital San Carlos de Denia Grupo HLA (Denia), Hospital Lluís Alcanys (Xátiva), Hospital General Universitario de Castellón (Castellón de la Plana), Hospital Francesc de Borja (Gandía), Vithas Perpetuo Internacional (Alicante), Hospital Psiquiátrico Penitenciario de Alicante y Centro Penitenciario (Alicante), Hospital General Universitario de Elche (Elche), Hospital Universitario y Politécnico La Fe (Valencia), Hospital Clínica Benidorm (Benidorm), Hospital La Malvarrosa (Valencia), Hospital General Universitario de Alicante, Hospital Arnau de Vilanova (Valencia). Extremadura: Complejo Hospitalario Universitario de Badajoz (Badajoz), Complejo Hospitalario de Cáceres (Cáceres), Hospital Virgen del Puerto (Plasencia). Galicia: Complexo Hospitalario Universitario de Pontevedra-Hospital do Salnés (Pontevedra), Hospital Álvaro Cunqueiro (Vigo), Complexo Hospitalario de Santiago de Compostela (Santiago de Compostela), Hospital Arquitecto Marcide (El Ferrol), Hospital Virxe da Xunqueira (A Coruña), Complexo Hospitalario Universitario de Ourense (Ourense), Centro Médico El Carmen (Ourense), Hospital Universitario Lucus Augusti (Lugo). Islas Baleares: Hospital de Llevant (Porto Cristo), Hospital Can Misses (Elvissa), Hospital Comarcal de Inca (Inca), Hospital Universitario Son Espases (Palma de Mallorca), Hospital Manacor (Manacor). Islas Canarias: Dr. José Molina Orosa (Arrecife de Lanzarote), Hospital Universitario Dr. Negrín (Las Palmas de Gran Canaria). La Rioja: Hospital Universitario San Pedro (Logroño). Comunidad Foral de Navarra: Residencia Mayores de San Adrián (San Adrián), Residencia Casa Misericordia (Pamplona), Centro San Francisco Javier (Pamplona), SF Sociosanitario del Servicio Navarro de Salud (Pamplona), Clínica Arcángel San Miguel (Pamplona), San Juan de Dios Residencia Landazábal (Burlada), Clínica Psiquiátrica Padre Menni (Pamplona), Residencia La Vaguada (Pamplona), Hospital Reina Sofia Tudela (Tudela), Complejo Hospitalario de Navarra (Pamplona). País Vasco: Clínica La Asunción (Tolosa), Hospital Universitario Basurto (Bilbao), Hospital Zamudio (Zamudio), QuirónSalud Bizkaia (Erandio), HUA Txagorritxu (Vitoria), Hospital San Eloy (Barakaldo), Hospital Alto Deba (Arrasate-Mondragón), Hospital de Zumárraga-OSI Goierri Alto Urola (Zumárraga), Hospital Santa Marina (Bilbao), Clínica Imq Zorrotzaurre (Bilbao), Fundación Onkologikoa (San Sebastián), OSI Bidasoa Hospital (Hondarribia), Hospital de Mendaro (Mendaro), Hospital Ricardo Bermingham (San Sebastián), Hospital de Galdakao-Usansolo (Galdakao), Hospital Urduliz-Alfredo Espinosa (Urduliz), Hospital San Juan de Dios Mondragón (Mondragón), Hospital Universitario Donostia (DonostiaSan Sebastián), Hospital Gorliz (Gorliz), Hospital Cruz Roja (Bilbao), Hospital San Juan de Dios (Santurtzi), Hospital Psiquiátrico de Álava (Vitoria-Gasteiz). Principado de Asturias: Fundación Hospital de Jove (Gijón), Hospital Universitario Central de Asturias (Oviedo), Clínica Asturias (Oviedo), Hospital Begoña de Gijón (Gijón), Instituto oftalmológico Fernández-Vega (Oviedo), Hospital Universitario de Cabueñes (Gijón). Región de Murcia: Hospital Clínico Universitario Virgen de la Arrixaca (Murcia), Hospital Nuestra Señora del Perpetuo Socorro (Cartagena), Hospital Universitario Rafael Méndez (Lorca), Hospital Los Arcos Mar Menor (San Javier).











 texto en
texto en 



