Introduction
Central venous catheters (CVC) are frequently used to administer antineoplastic treatment in oncology patients. However, its usage is not without complications, mainly due to catheter-related bloodstream infections (CRBSI) which have associated mortality of approximately 12-25%1, 2. There are frequently produced by gram-positive bacteria (75%)1,3. Furthermore, since 80% of coagulase-negative Staphylococcus (CNS) strains are meticillin-resistant and the increase in the prevalence of resistant Staphylococcus aureus to meticillin (MRSA), vancomycin and daptomycin are the treatment of choice for CRBSI2,4,5. Even though both antibiotics have shown to be effective in the treatment of CRBSI, to date, there are only a small number of comparative studies addressing its effectiveness and safety6.
This study aims to analyse the effectiveness and safety of daptomycin versus vancomycin on CRBSI in patients with solid tumors in routine clinical practice.
Methods
Study design and patient selection criterio
This retrospective study evaluated the effectiveness and safety of vancomycin, compared with that of daptomycin in patients with solid tumors with gram positive CRBSI. Oncologist Subjects with gram positive CRBSI who were hospitalized over 8-years period (2010-2018) at a 822-bed tertiary care hospital in Tenerife, Spain, were eligible for inclusion. Eligible patients were aged ≥ 18 years with solid tumor with Gram-positive CRBSI, without a source for the bacteraemia other than the CVC who were treated either vancomycin or daptomycin. Empirical therapy was considered when an antimicrobial regimen was administered within 24 hours of extraction of the blood sample, and before susceptibility was known. Patients received vancomycin or daptomycin according to treating clinician preference.
Patients were ineligible if they had any one of the following: patients with hematologic malignancies, neutropenic patients, no etiological agent identified or confirmed Gram-negative CRBSI. Patients in whom the antibiotic treatment was modified were excluded.
Because of the retrospective observational design of the study, neither patient consent nor ethics approval was required at the time the study was carried out.
Clinical variables evaluated
The study outcome was evaluated considering: demographic data and Charlson Comorbidity Index, which provides a general measure of severity of disease7. Antibiotic dose, frequency, and duration were recorded. To evaluate safety, nephrotoxicity was defined as an increase in the serum creatinine level of 0.5 mg/dL or 50%, whichever was greater, on at least two consecutive measurements from the initiation of antibiotic to 3 days after treatment8. Creatine phosphokinase values were evaluated in daptomycintreated subjects. A clinically significant elevation was defined as 5 times the upper limit of normal (ie, 0.850 U/L).
Antibiotic susceptibilities were confirmed by broth microdilution, according to the Clinical and Laboratory Standards Institute (CLSI) guidelines9. Clinical categories were determined according to the breakpoints defined by the European Committee on Antimicrobial Susceptibility testing (EUCAST) criteria10 and CVC were removed in patients with documented CRBS due to S. aureus.
Definitions and outcome assessment
CRBSI was classified according to the current Infectious Diseases Society of America (IDSA) criteria guidelines5.
The primary end point, was defined as a composite of 30-day mortality rate (M30), the re-admission rate at 30 days (R30) and the length of hospital stay (LOS). 30-day mortality was defined as mortality occurring in the 30-day period following index culture, and the R30 as the re-admission of the patient caused for any reason within the 30 days of index culture.
Statistical análisis
Statistical analysis was conducted using the Chi-squared test for the comparison between M30 and R30 from both therapeutic groups with SPSS Statistics v. 25.0 software (IBM Corporation, Armonk, NY, USA). To study the nephrotoxicity from the different groups the Chi-squared test was also the statistical test selected. The differences between the basal characteristics in both groups of patients were evaluated by the Mann-Whitney U test for that continuous quantitative variables and alternatively using a Chi-Squared for the dichotomous variables. The statistical significance was established as p < 0.05.
Results
Demographic characteristics of the study population
A total of 558 cancer patients with an episode of suspected of CRBSI were treated with either vancomycin or daptomycin. Among them, 70 met the inclusion criteria, 47.1% (n = 33) were male and their average age 57.9 years old (standard deviation [SD] = 11.5). The 61.4% (n = 43) of these patients received vancomycin and the 38.6% (n = 27) daptomycin as a treatment.
The baseline characteristics of the two groups are presented in table 1; there were no significant differences in baseline characteristics between the two groups, differing only in the percentage of infections caused by the meticillin-susceptible Staphylococcus aureus (MSSA) which was higher in the group of patients treated with daptomycin (33.3% [n = 9] versus 11.6% [n = 5]; p = 0.027) (Table 2).
Table 1. Patient demographic characteristics
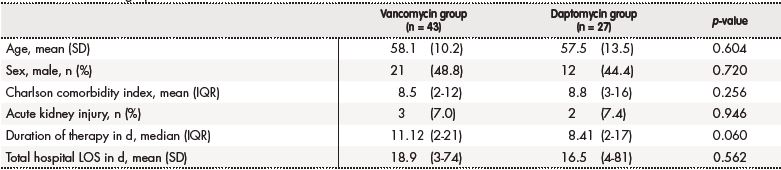
d: number of days; IQR: interquartile range; LOS: length of stay; N: number of patients; SD: standard deviation
Table 2. Characteristics of the microorganisms causing central intravascular catheter infections
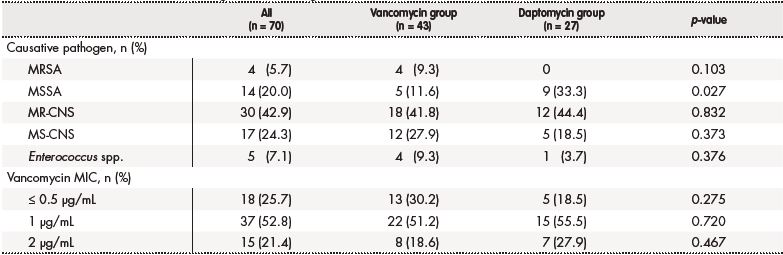
MIC: minimum inhibitor concentration; MR-CNS: meticillin-resistant coagulase-negative staphylococci; MRSA: meticillin-resistant Staphylococcus aureus; MS-CNS: meticillinsusceptible coagulase-negative staphylococci; MSSA: meticillin-susceptible Staphylococcus aureus; n: number of patients
The pharmacokinetic monitoring of vancomycin plasma concentrations was determined only in the 23.3% (n = 10) of the patients (average dose: 14.1 mg/kg/12 h; interquartile range [IQR]: 1.8-19.6). The average daily dose of daptomycin was 568.7 mg (average dose: 7.43 mg/kg/day; IQR: 4.2-12.3).
Effectiveness evaluation
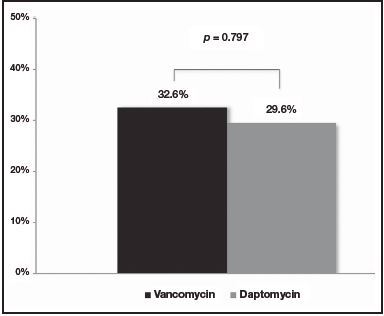
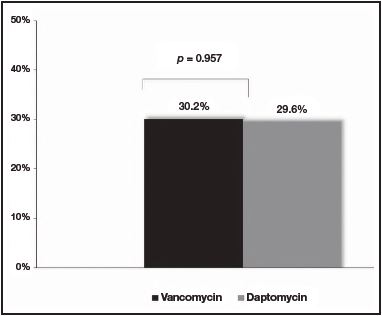
No differences were observed at the M30 between patients treated with vancomycin and daptomycin (32.6% [n = 14] versus 29.6% [n = 8], respectively [p = 0.797])(Figure 1). Additionally, no differences were found at the R30 (30.2% [n = 13] for vancomycin group versus 29.6% [n = 8] for daptomycin group [p = 0.957]) (Figure 2). In the same line, LOS was similar between the two treatment groups (18.9 days [IQR: 3-74] for vancomycin group versus 16.5 days [IQR: 4-81] for daptomycin group [p = 0.562]) (Table 1).
Safety assessment
Nephrotoxicity rate was similar in both groups of treatment: 7% (n = 3) of the patients treated with vancomycin versus a 7.4 % (n = 2) of the daptomycin group (p = 0.946) (Table 1). One patient treated with vancomycin suffered kidney failure and two developed kidney injury. In the case of daptomycin group, two patients showed kidney injury. No significant elevation of Creatine phosphokinase were observed in our cohort of patients.
Discussion
Few studies address a direct comparison between vancomycin and daptomycin as the treatment for CRBSI in oncology patients6. Therefore, the best therapeutic alternative in these patients remains still under discussion.
The results of our study show that, in routine clinical practice, vancomycin presents similar effectiveness and safety to daptomycin in the treatment of these infection. Moreover, the equivalence in effectiveness shown in this analysis has been achieved despite the use of infratherapeutic doses of vancomycin for most patients and without a pharmacokinetic optimization of the dosing regimen in 76.6% of cases, as currently recommended11.
Chaftari et al. carried out the only study compared both therapeutic alternatives in cancer patients with CRBSI6. In this study, a cohort of 38 cancer patients with either suspected or confirmed CRBSI treated with daptomycin were compared with a historical cohort of 40 patients treated with vancomycin. Analysed the clinical results of in their study, daptomycin showed faster bacteriological eradication and clinical resolution.6
In contrast, our study demonstrating a lack of benefit for daptomycin over vancomycin in oncologist patients with confirmed gram-positive CRBSI. We might speculate that the different result between our study and Chaftari’s one might be attributed, at least in part, to differences in the bacteria strains isolated. In our study, the 78.5% of the isolated bacteria showed a vancoymicin MIC ≤ 1 μg/mL, while Chaftari’s and colleges found that the 74.3% of the Staphylococcus strains showed a vancomycin MIC between 1-2 μg/mL6.
This study was conducted at a single institution and was of a retrospective nature, which may limit its generalizability to other settings. These results will require validation in a prospective, randomized, controlled comparative efficacy trial. However, variables such as the Charlson index, which integrates information on factors related to the comorbidity and severity of the disease of different patients, were taken into account in order to reduce possible biases. Thus, we tried to make the baseline characteristics of both treatment groups as balanced as possible.
The present study represent at this moment, the largest cohort of oncologist patients with confirmed CRBSI, comparing the effectiveness and safety of both therapeutic alternatives. Our results show the equivalence, in safety and effectiveness, between both antibiotics. As a result, we conclude vancomycin should remain the treatment of choice for these infections, especially in centres where the prevalence of strains with decreased susceptibility to vancomycin is low.











 text in
text in