Introduction
QT interval prolongation may result in a lengthening of the patients' hospital stay and in an increase in morbidity and mortality1-3. Moreover, many drugs are available which are able to prolong the QT interval and increase the risk of torsade de pointes (TdP) tachycardia, particularly if they are used in patients with additional risk factors2,4,5.
Several procedures have been developed to prevent QT interval prolongation, which are based on drug therapy, potentially correctable factors and structural diseases or hereditary genetic arrhythmias6,7. When a prolonged QT interval is detected, the patient must be evaluated and all the medicines in their drug regimen able to prolong the QT interval must be discontinued6-8. The reduction of the incidence of QT interval prolongation at hospital level requires pharmacotherapeutic and electrocardiographic monitoring and an assessment of the risk factors presented by each patient. Implementation of a protocol aimed at preventing QT interval prolongation and the complications associated to is also necessary.
This study seeks to determine the incidence of patients at risk of presenting with treatment-associated QT interval prolongation in a private hospital in Costa Rica, evaluate the drugs prescribed to treat the condition and the most common clinical risk factors faced by patients. Moreover, an análisis was made to determine which QT interval measurement method (manual or automatic) and which of the available adjustment or correction formulae (QTc) are best suited to electrocardiographically monitor hospitalized patients.
Methods
A retrospective observational study was carried out of patients admitted to the Clínica Bíblica Hospital, a 78-bed private center located in San José, Costa Rica.
An analysis was conducted of the clinical records of all patients over 18 years of age hospitalized for over 48 hours and administered at least one drug from list 1 (medicines associated with QT interval prolongation) or two or more drugs from lists 2 and 3 of the CredibleMeds classification9. These lists enumerate medicines capable of prolonging the QT interval that are clearly associated with TdP (list 1); medicines capable of prolonging the QT interval but where an association with TdP has not been demonstrated (list 2); and drugs that have been shown to result in TdP only under certain usage conditions (list 3) such as the administration of excessive doses, hypokalemia, concomitant use of drugs inhibiting the metabolism of QT interval prolonging medicines, and TdP-inducing electrolytic disorders.
Patients with pacemakers and those whose clinical records lacked the information minimally required for our analyses were excluded from the study. Data was gathered both from the patients' electronic medical records and from paper-based records by a group of pharmacy students supervised by their professors and by clinical pharmacists. The MPH Hospitalized Patients Management System, the SIH Integrated Hospital Management System and the SIFA Pharmacy System were reviewed.
The following information was obtained: (1) general characteristics (age sex, diagnosis on admission, length of hospital stay); (2) drugs associated to QT interval prolongation (active ingredient, route, dose); (3) existing drugdrug interactions; (4) clinical factors associated to QT interval prolongation (RISQ-PATH score)10; (4) electrocardiographic data (ECG result, QTc value [obtained manually or automatically], among others) and (5) management in the event of arrhythmias and electrocardiographic disorders. The RISQPATH score was used with a cutoff value of 10 points. Patients with higher scores were classified as patients at a high risk of suffering QT interval prolongation during their stay in hospital10. The assessment of risk was carried out using the RISQ-PATH scale.
QT values had been measured in duplicate using standard resting 12-leand ECGs (paper speed: 25 mm/s and a gain setting of 10 mm/mV). Electrocardiographic data (QRS, QT, RR) were determined both automatically and manually (using the tangent method) in leads II or V, as required, to carry out a comparison that made it possible to determine whether the difference between the values obtained with both methods (manual or automatic) was statistically significant (unpaired Student's t test).
Manually-measured QT intervals were corrected to QTc using Fridericia's (if QRS < 120 ms) and Rautaharju's (if QRS > 120 ms) formulae and automatically-measured QT intervals were corrected using Bazett's formula11,12.
In addition to the foregoing, the variability between the manual and automatic methods was analyzed. In the case of patients with atrial fibrillation, the same correction formulae were applied, with the RR interval variant, which was duly corrected13,14.
Drug-drug interactions were analyzed suing the Micromedex and UpTo-Date databases.
A paired Student's t test was carried out to determine whether there was a significant difference between the QT interval as measured on admission and the QT interval as measured on the follow-up ECG. Data was analyzed using the latest version of Microsoft Excel as well as SPSS v25 for Windows.
The lead investigators guaranteed the ethical use of data as well as the anonymity of all information. Both the Clínica Bíblica Hospital and the Ethical-Scientific Committee of the University of Costa Rica gave their authorization for the performance of this research.
Results
Between January and December 2018, a total of 540 patients were admitted to the Clínica Bíblica Hospital, of whom 141 fulfilled the inclusión criteria. Of them, 30 (21.2%) were either currently afflicted by some kind of arrhythmia or electrocardiographic disorder (17; 12%) or had experienced such conditions in the past (23; 16.3%). Fourteen of the latter (9.9%) experienced a complication of their arrhythmia during their stay in hospital. Electrocardiographic disorders included atrial fibrillation, left branch bundle block, extrasystole, trigeminy and a side effect potentially associated with the use of digoxin. The characteristics of patients included in the study can be seen in table 1.
Table 1. Characteristics of the population included in the study
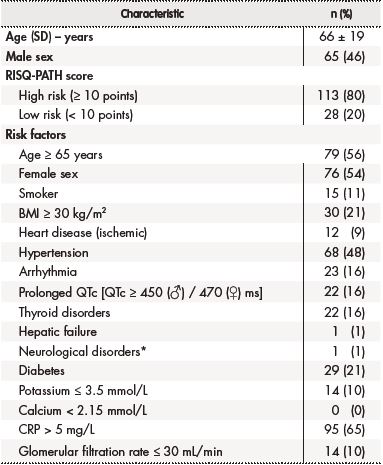
*Stroke.BMI: body mass index (kg/m2); QTc: corrected QT interval; SD: standard deviation.
Of the total of high-risk patients (113), only 64 (56.6%) were subjected to an ECG on being admitted to the hospital. For 25 (39.0%) of them, at least one follow-up ECG was found which allowed a comparison of the QT interval before and after indication of a pharmacological therapy. In this population, it was found that 10 (15.6%) patients presented with a delta QT value (difference between the follow-up QT and the QT on admission) of ≥ 30 ms. Four of these patients (40%) exhibited a difference of over 60 ms, which is considered a severe QT interval prolongation, denoting a much higher risk of experiencing life-threatening cardiac events.
On the other hand, 62 (54.8%) of the 113 patients were considered high risk even before being treated in hospital; 22 (35.5%) of them presented with QT interval prolongation during their stay in hospital and in 5 (8.0%) of them the risk was associated with the use of such drugs as quinolones (moxifloxacin and ciprofloxacin) and fluconazole, which are clearly connected with the development of TdP.
The medicines associated with an increased risk of QT interval prolongation, and which were prescribed to hospitalized patients included levosulpiride (58%), ondansetron (56%), amiodarone (19%), metronidazole (8%) and quetiapine (2%). The mean number of high-risk drugs prescribed per patient was 3 (1-10), with 8% of patients receiving as part of their therapy six or more drugs related to QT interval prolongation of any grade and to the appearance of complex ventricular arrythmias (Table 2).
Table 2. Arrhythmias occurring during hospitalization and treatment
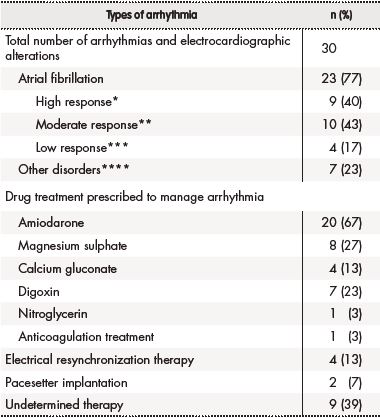
*High response: heart rate ≥ 100 beats/min.
**Moderate response: heart rate 51-99 beats/min.
***Low response: heart rate ≤ 50 beats/min.
****Definition: Right branch bundle block, left branch bundle block, ventricular tachycardia, sinus tachycardia or ventricular extrasystole.
Twenty-seven patients (19.1%) experiences over four clinically significant drug-drug interactions apt to increase the risk of QT interval prolongation. The most involved drugs included ondansetron, levosulpiride and amiodarone. No instances of TdP or complex ventricular tachycardia were observed, and none of the patients died while in hospital.
A comparison of the manual measurement of the QT interval with the values provided by electrocardiography showed a significant difference between both (p = 0.0004). The corrected QT interval (QTc) obtained using both methods were also compared, with manually obtained values being superior to automatically-obtained ones.
When corrected using Bazett's formula, QTc intervals were longer tan when Rautaharju's formula was used (p ≤ 0,0001). No significant difference was found between corrections with the Bazett and the Fridericia formulas, but a significant difference was observed between corrections made with Fridericia's formula and those where Rautaharju's formula was used (p ≤ 0,0001).
Discussion
QT interval prolongation tends to be closely connected with the appearance of ventricular arrhythmias such as TdP, which could even result in sudden death. Similarly, this electrocardiographic alteration has been associated to longer hospital stays and to increased mortality due to cardiovascular events15-18.
The incidence of QT interval prolongation is generally high among hospitalized patients. In an analysis of 422 elderly adult hospitalized patients, the alteration was identified in 32% of cases17, while another study conducted at the emergency room of a third-level hospital, 95 (34.1%) of 279 patients subjected to an ECG presented with QT interval prolongation, with 15% of them exhibiting a QT interval over 500 ms19. In line with the literature, the present study found that 31% of ECGs performed on admission revealed some degree of QT interval prolongation.
This study made use of the RISQ-PATH score to stratify patients according to their level of risk. The score was developed based on a systematic review of clinical evidence with respect to the risk factors most associated with QT interval prolongation. This score provides a sensitivity of 96% and a negative predictive value of 98%, which demonstrates its utility in identifying cases where a follow-up ECG is not required10.
Eighty percent of patients in the analyzed sample presented with a high QT interval prolongation risk, which depended on their individual characteristics and the pharmacological therapy prescribed. Fifty-six percent of patients (62) were found to have a high QT interval prolongation risk before their admission, i.e. prior to their therapy being prescribed. This shows the importance of identifying patients at risk as early as posible20.
Several studies have shown that the likelihood of developing TdP as a result of the administration of drugs that increase the risk of QT interval prolongation is significantly higher in hospitalized patients. This can be explained by the fact that this patient population tends to be older than average, which was the case of the patients in this study, where mean age was 66 years. Moreover, this kind of patient usually presents with additional risk factors such as electrolytic disorders and hepatic and renal dysfunction21.
Hypokalemia is a risk factor that was observed in 10% of the patients analyzed. The presence of hypokalemia on admission by itself trebles the risk of QT interval prolongation22. Only half of the patients in this study were subjected to an ECG on admission, two of them presenting with a prolonged QT interval with values of 486 and 606 ms.
Given the high incidence of this kind of electrocardiographic alterations and the severity of the resulting complications, some hospitals have introduced an alert system capable of analyzing each of the ECGs performed and raises an alarm when abnormal values are obtained23. According to the American Heart Association, a useful way of preventing these events is to carry out an exhaustive analysis of the risk factors associated with QT interval prolongation in order to rapidly identify cases where stricter electrocardiographic monitoring may be required and to minimize the incidence of risk when selecting the patients' medication20.
Different analyses have pointed out that, because of the long list of drugs associated with a risk of QT interval prolongation, electrocardiographic monitoring it not feasible in all patients treated with these drugs. However, in patients presenting with some kind of arrhythmia during their hospital stay, or in those with multiple risk factors, such monitoring should be performed in all cases as it is considered to be the most efficient way of preventing adverse events10,24
The use of more than one QT interval prolonging drug is a risk factor. In a recent study, 48% QT interval prolongation events were attributable to the medication and involved two or more high risk medicines in 25% of cases25,26. The high number of drugs that were on average prescribed to the patients in this study could be related to the observed incidence of QT interval prolongation. At any event, other treatment alternatives should be evaluated.
Another factor that should be borne in mind when prescribing QT Interval prolonging medicines is the relationship between the drugs' plasma concentration and the effect this concentration has on risk. Fourteen patients (12%) of the sample analyzed presented with clearance rates below 30 mL/min, with three patients being prescribed higher doses than recommended or required as a function of the patients' glomerular filtration rate27.
Measurements of the QT interval were made both manually and automatically. The latter methods are not always accurate and require manual validation of the results obtained, particularly in patients with electrocardiographic abnormalities such as early repolarization and arrhythmia (including atrial fibrillation)28,29.
Another limitation associated with automatic determination of the QT interval has to do with correcting potential prolongations. Indeed, even if the most commonly used method is Bazett's formula, previous studies have questioned the value of this method, suggesting that Fridericia's method should be the correction method of choice11. The shortcomings of Bazett's formula have been extensively documented in other studies. It is to be expected for some formulae to be more appropriate than others depending on specific characteristics of the studied population such as their heart rate, their sex, their ethnicity, and their age30.
In patients with a QRS complex ≥ 120 ms, it was deemed necessary to use Rautaharju's formula to correct the QT interval as a statistically significant difference was observed between Rautaharju's formula and Bazett's and Fridericia's formulae. This could be attributable to the fact that Rautaharju's is the only formula that takes heart rate fluctuations into consideration when making the correction15,16.
The fact that this is a descriptive study conducted in a retrospective manner based on the analysis of clinical records constitutes a limitation as many of the patients' clinical records did not include the required information. Moreover, it must be noted that this study was conducted in one single hospital serving a small population, which means that the information obtain cannot be generalized to other populations or health centers.
The results obtained from this study indicate that the risk of drug-induced QT interval prolongation is fairly common, making it necessary to implement strategies conducive to more effectively monitoring and correcting the QT interval (QTc) in order to prevent the complications associated with prolongation. Every effort should be made to mitigate preventable risk factors avoiding QT interval prolonging drugs, excessive dosing of high-risk drugs or abnormal electrolyte levels.
In addition, institutional protocols must be implemented that promote ECG-based monitoring of patients at risk or who suffer from diseases associated to electrocardiographic disorders as the onset of TdP and other ventricular tachycardias may be prevented if a growing QT interval is detected on time21,31.











 texto en
texto en 


