Introduction
Delay in the approval and marketing of new pharmaceuticals, especially in the field of Medical Oncology, can imply that promising drugs are not authorised, in spite of existing evidence base supporting their use. As a consequence, drugs in special situations have emerged as a treatment option that allows the use of an unauthorised medicine1. The level of evidence supporting off-label drug use in clinical practice has been scarcely addressed in the literatura2.
Over the years, compassionate use has evolved to become a very complex issue involving pharmaceutical companies, regulatory agencies, physicians, patients and patient advocacy groups3.
The use of drugs in exceptional circumstances refers to the use of nonauthorised medicines or the use of medicines outside their authorised conditions, and includes:
Off-label use: the use of an authorised medicinal product for an indication different from those provided for product characteristics
Compassionate use: the use of investigational drugs (unauthorised) in patients with no satisfactory authorised therapies and who cannot enter RCTs
Foreign drugs: the use of medicines unauthorised in Spain but authorised in other countries4
The Spanish legislation limits the use of each of these criteria to those exceptional circumstances in which there is no other comercial therapeutic alternative. The process of drugs in exceptional circumstances' authorisation in case of unauthorised drugs in our country (compassionate use or foreign drugs) has multiple steps (in sequence): a) the informed consent of the patient; b) the request of a specialist physician; c) the agreement of the Medical Chief Director of the healthcare centre and, finally d) authorisation by the Spanish Agency of Medicines and Medical Devices4. In some cases, the agreement of the promoter or pharmaceutical company is also required. In the case of the off-label use, authorisation from the Spanish Agency is not required. The physician must adequately justify in the clinical history the need for the use of the medicine and inform the patient of the possible benefits and potential risks.
Article 6 of Directive 2001/83/EC1 requires that medicinal products are authorised before they are marketed in the European Community5. This article formulates only two general requirements for compassionate use: 1) a chronically or seriously debilitating disease, or a life threatening disease of patients who cannot be treated satisfactorily with an authorised medicinal product, and 2) the medicinal product must be either the subject of an application for a centralized marketing authorisation or be undergoing clinical trials3. Compassionate use (CU) programmes are coordinated by Member States, which set their own rules and procedures6.
A literature review explored compassionate use in 28 EU member states, concluding that compassionate use program (CUP) was present in 20 EU member states (71%). Of 28 EU states, 18 had nationalized regulations and processes were well-defined7.
Patients should always be considered for inclusion in randomized clinical trials (RCTs) before being offered compassionate use programmes. RCTs are practically the best means of obtaining reliable and interpretable efficacy and safety data for a medicinal producto5.
In 2014, the number of requests for expanded access to investigational new drugs received by the Food and Drug Administration increased by twofold compared to those received in 2005. Anti-cancer drugs represented approximately a quarter of the applications. Overall, 99.7% of the submitted requests for expanded access were accepted8.
The aims of this study were:
– To analyse the applications for drugs in special situations for solid tumours in a representative Spanish third-level centre, describing the authorised drugs (generic names) and their indications
– To assess the level of evidence supporting these applications
– To evaluate effectiveness and safety of most frequent drugs used in special situations
Methods
Study design
We conducted a cross-sectional study of all applications for drugs in special situations during 2018 and 2019 in a representative third-level hospital. All drugs in special situations were identified and of these, the drugs used for solid tumours were selected to perform this study.
Variables
We collected data about generic names of drugs, indications, and level of evidence provided (according to the hierarchy of study types and its correlation to levels of clinical evidence established by National Health and Medical Research Council [NHMRC] and National Institute for Clinical Excellence [NICE]: animal and laboratory studies, case report or case series, observational studies, and RCTs —divided into three phases—).
The baseline characteristics of patients, such as age and Eastern Cooperative Oncology Group (ECOG) performance score, were analysed. Tumour response was assessed using the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1, Progression Free Survival (PFS) and Overall Survival (OS). Safety was evaluated with the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. We also collected data about time to adverse effect and need for change of treatment. Finally, we discussed and compared our real-world experience data with those published from RCTs.
Data collection and drug approvals
Data were obtained from the database of drugs in special situations recorded by the drug information centre of the Hospital Pharmacy department (software PKusos® https://www.pksiam.com/service/pkusos/)9
Before authorizing the drug used in special situation, a multidisciplinary eam evaluates the available evidence on its use in this special situation. Each submitted application was considered on a case-by-case basis. An ad hoc Hospital Committee evaluated whether each request met the criterio to be used as a medicine in exceptional circumstances. Those drugs evaluated by the Hospital Committee with a positive assessment of the submitted application for antineoplastic drugs in a special use situation were collected.
Statistical analysis
Statistical analysis was performed using Stata (developed by Stata-Corp), and the MS Excel (Microsoft) was used to create figures and charts. Frequencies and percentages were used for categorical variables and means (standard deviations, SD) or medians (interquartile ranges, IQR) for continuous variables, depending on the distribution of the variable. Survival was analysed using Kaplan-Meier curves.
Results
Overall, 2,273 drugs in special situations were approved between January 2018 and December 2019 (99.5% of total applications). In 431 (19%) applications, the diagnosis was a solid tumour.
Regarding the frequency distribution of departments which requested for drugs in special circumstances, the most common clinical department was ophthalmology with 440 applications (19.3%), followed by oncology —431 (18.9%)— and hematology —289 (12.7%).
Table 1 shows the clinical indications of drugs in special situations for solid tumours and level of evidence provided at the moment of application.
Table 1. Frequency of clinical indications (types of tumours) and level of evidence of medicines in special circumstances provided at the moment of application

CRC: colorectal cancer; CRPC: castration-resistant prostate cancer; RCC: renal cell carcinoma.
Hepatocellular carcinoma (13.4%), lung cancer (13.2%) and breast cancer (12%) were the most treated pathologies using drugs in special situations. We obtained information about the level of evidence provided at the moment of application in 365 cases (84.9%). The majority of drugs in special situations were supported by phase 3 (47%) o phase 2 (33%) trials (Table 1).
Out of 431, 291 (67.5%) applications for solid tumours were off-label drugs, 76 (18%) foreign drugs, and 64 (15%) were compassionate use of drugs. The table 2 summarizes data about drugs in special situations for solid tumours.
Table 2. Off-label use (OLU), compassionate use (CU) and foreign drugs (FD) approved for solid tumours between 2018 and 2019
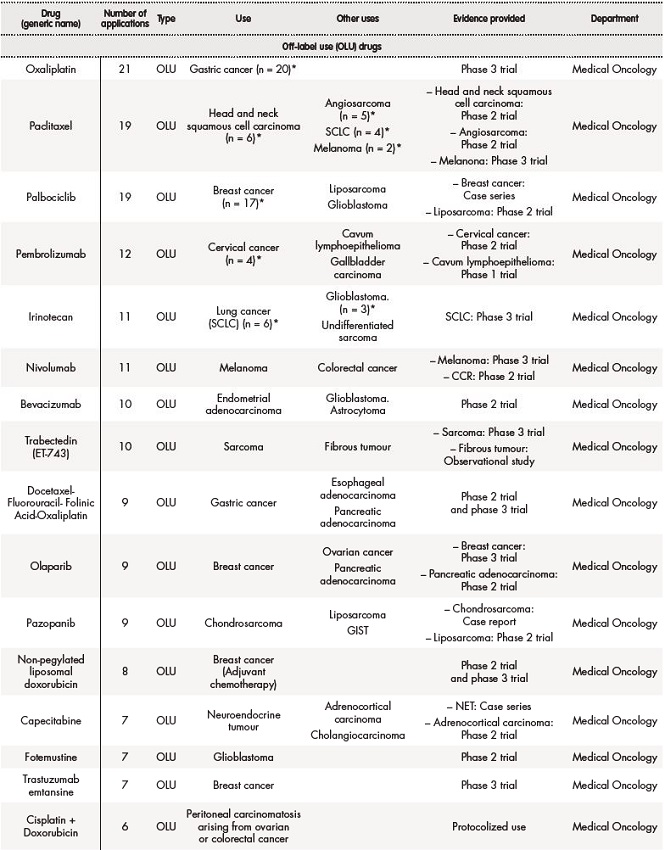
Table 2 (cont.). Off-label use (OLU), compassionate use (CU) and foreign drugs (FD) approved for solid tumours between 2018 and 2019
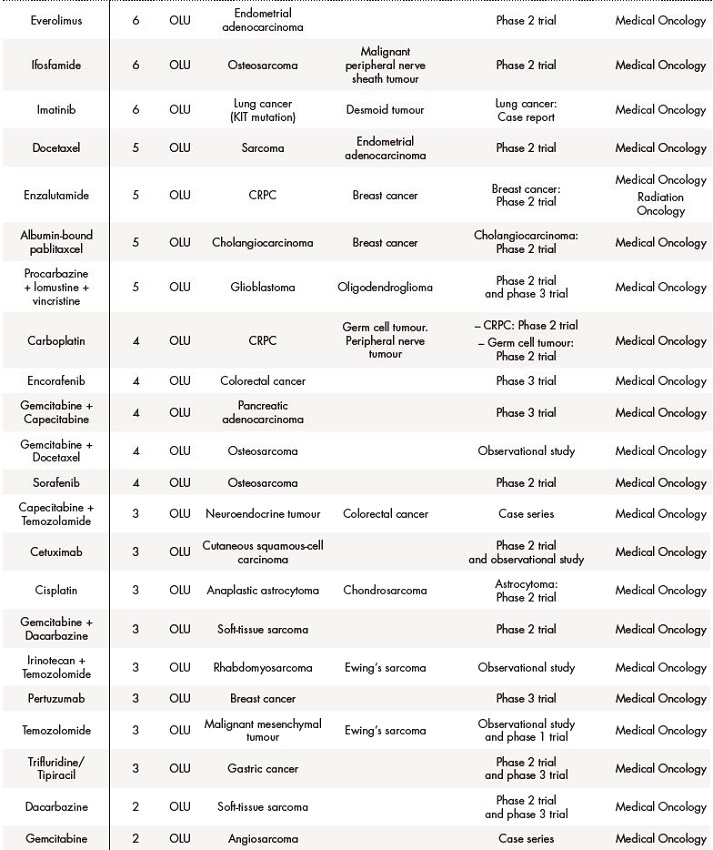
Table 2 (cont.). Off-label use (OLU), compassionate use (CU) and foreign drugs (FD) approved for solid tumours between 2018 and 2019
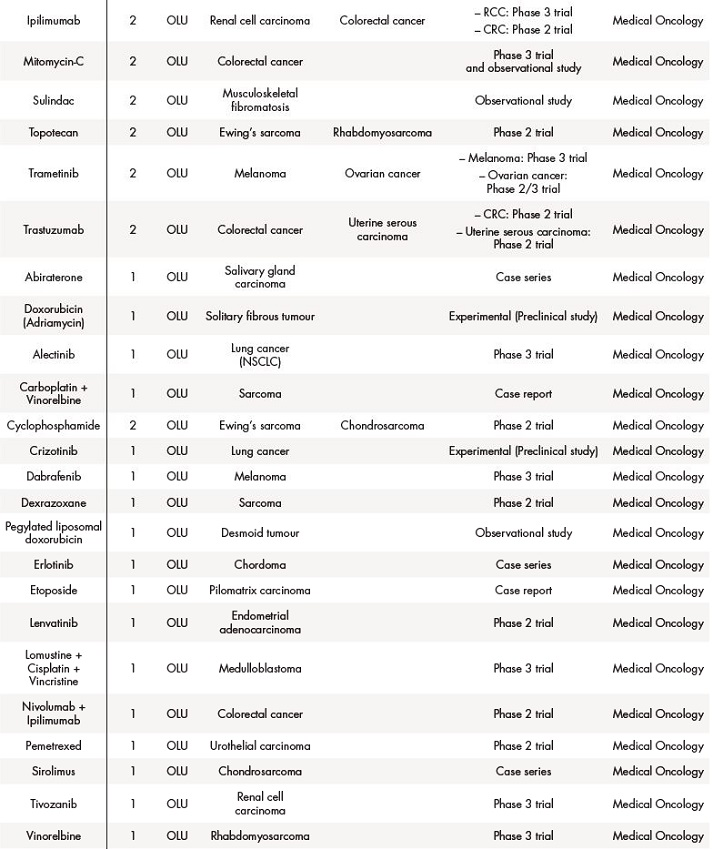
Table 2 (cont.). Off-label use (OLU), compassionate use (CU) and foreign drugs (FD) approved for solid tumours between 2018 and 2019
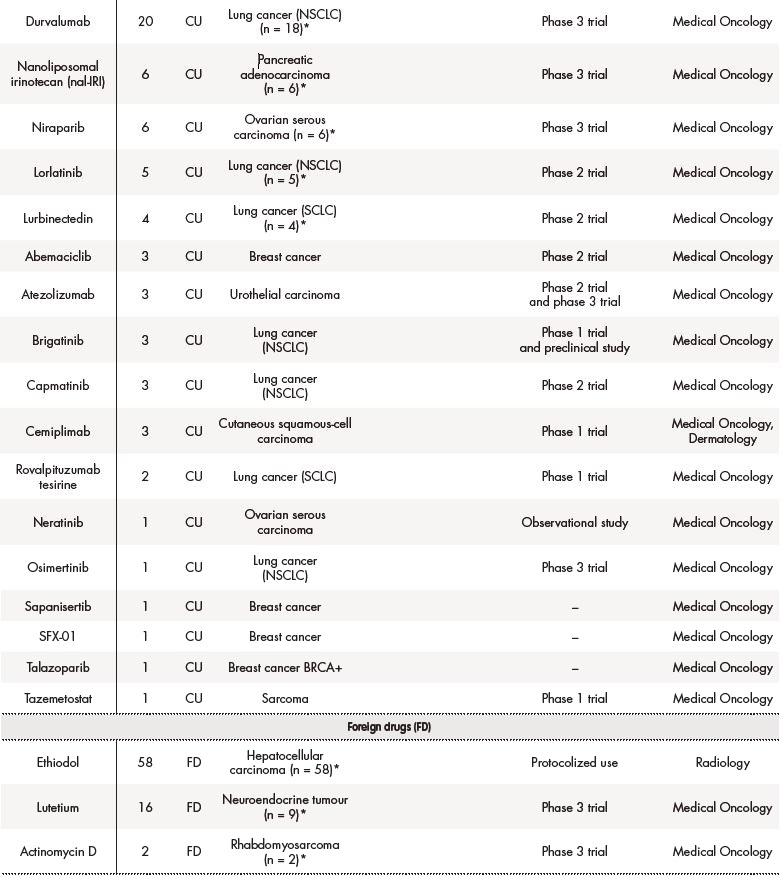
The asterisk (*) indicates that their effectiveness and toxicity profiles were analysed (see Table 3). Some missing values were detected (follow-up in other centres, deaths before the CT evaluation…).CRC: colorectal cancer; CRPC: castration-resistant prostate cancer; GIST: gastrointestinal stromal tumour; NET: neuroendocrine tumour; NSCLC: non-small-cell lung carcinoma; RCC: renal cell carcinoma; SCLC: small cell lung cancer.
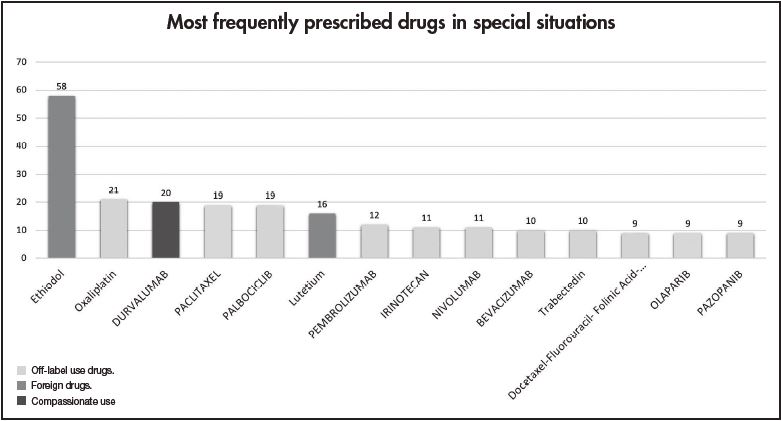
Figure 1 shows the most frequent drugs (generic names) in special situations for solid tumours during 2018-2019. Ethiodol, oxaliplatin and durvalumab were the most commonly prescribed foreign, off-label use and compassionate drugs, respectively.
Table 3 contains the data regarding effectiveness and safety of most commonly prescribed drugs in special situations during the study period. Some missing values were detected (follow-up in other centres, deaths before the CT examination, etc.). Real-world RECIST–based complete or partial responses were found in 28.6% of patients treated with oxaliplatin for gastric cancer, 40% of patients diagnosed with squamous cell carcinoma treated with paclitaxel, 7.7% of palbociclib uses in breast cancer patients, and in 33.33% of patients with cervical cancer who were treated with pembrolizumab. The majority of toxicities were grade 1 according to CTCAE 5.0 and only in 6/67 cases the treatment was discontinued due to adverse effects.
Table 3. Real-life experience on effectiveness and safety of the most frequently prescribed drugs in special situations.

Table 3 (cont.). Real-life experience on effectiveness and safety of the most frequently prescribed drugs in special situations.
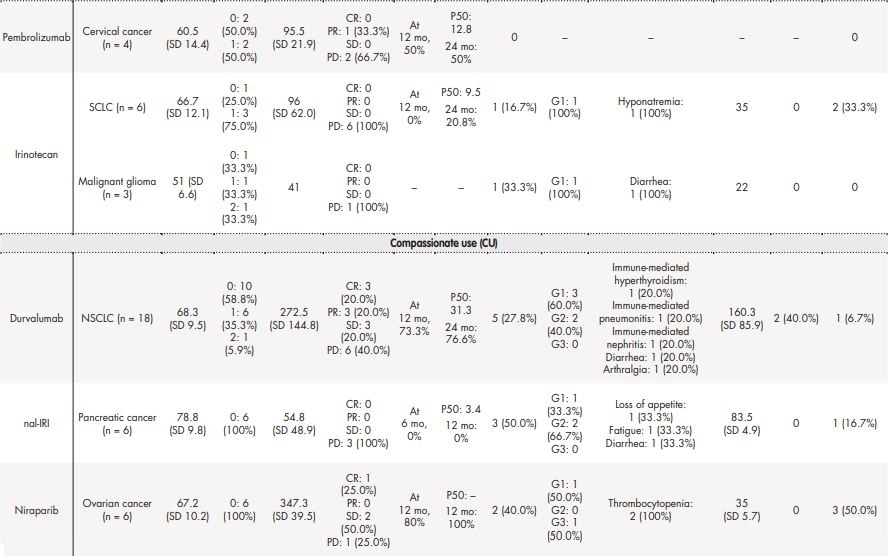
Table 3 (cont.). Real-life experience on effectiveness and safety of the most frequently prescribed drugs in special situations.
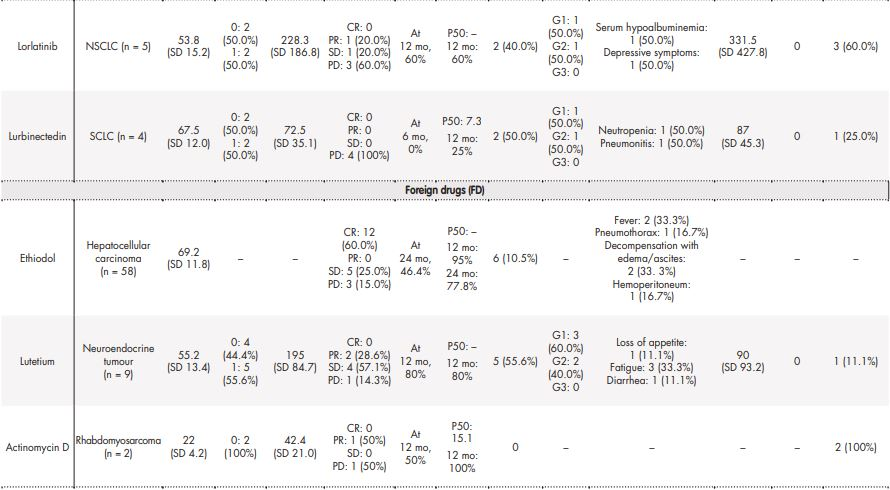
CR: complete response; G1: grade 1; G2: grade 2; G3: grade 3; P50: median OS (overall survival); PD: progressive disease; PR: partial response; SCLC: small cell lung carcinoma; SD: stable disease.
Discussion
Evidence supporting use of drugs in special situations
The level of evidence supporting drugs in special situations is relevant because it is closely related to the expected effectiveness and safety of such treatments. Our study can be used to assess the role of the level of evidence in the decision of application for unathorised drugs for solid tumours.
Despite being considered as an important factor in the use and approval of medicines in exceptional circumstances10, only a few papers have focused on the evidence that supports the applications of these drugs. Nevertheless, there are several studies analysing the specific drugs approved as “special situation” use. Furthermore, no guidances have been specifically developed to help clinicians assess appropriateness in off-label prescribing. Gazarian et al. proposed a classification in order to guarantee the appropriate off-label use: off-label use justified by high-quality evidence, use within the context of a formal research proposal, and exceptional use, justified by individual clinical circumstances11.
The Spanish Society of Hospital Pharmacy (SEFH) published a survey in 2015 on the use of off-label drugs for Oncohematology patients in Spanish hospitals10. The survey showed that the main factor influencing the authorisation-prescription process of these drugs is the available evidence. Nevertheless, a lower level of evidence is usually accepted in cases in which there are no therapeutic alternatives, or in patients with low-prevalence tumours4. Also in Spain, Blanco-Reina et al. conducted a cross-sectional study in order to determine the level of evidence (according to the criteria by SIGN-NICE) supporting off-label drugs prescriptions in a third-level hospital during 2010. They report 190 applications for off-label prescription and 52.4% were based on some clinical trial, while the rest had a low level of evidence (observational studies and case reports)12. In contrast, in our centre 83.1% of drugs in special situations was supported by a RCT. Many reasons could be identified to explain these data. For example, the good level of implementation of drugs in special uses' programs in our context. Furthermore, the existence of unmet therapeutic needs and the rising level of convincing evidence recently, including the growth of adequate and well-controlled trials. Indeed, the proposal and acceptance of applications supported by a high level of evidence could be considered as a sign of proper functioning of these programs.
A study conducted by M.D. Anderson Cancer Centre researchers reported that a third of patients with metastatic breast cancer had received off-label therapy at some point during treatment13. Furthermore, an Italian multicenter study revealed that the off-label use in Oncology represented almost 20% of prescriptions, even if most of them were based on scientific evidence of efficacy (one or more RCTs or more phase II trials published in a relevant oncology journal). Addionally, the drugs prescribed were used in a different cancer (46.2%) or for a rare neoplasm (13.6%)14.
Use of drugs in special situations in cancer patients
Nowadays, 20% of drugs are used off-label, and the percentage is higher in cancer patients. Reasons for the off-label use of drugs in cancer patients include: the wide variety of cancer subtypes, the low prevalence of some tumours, the lack of alternative treatment options, difficulties in enrolling patients in clinical trials, the rapid diffusion of the preliminary results of drug RCTs, and delays in the approval of new drugs by regulatory agencies4,15.
Only 8% of NCCN guidelines are based on level I evidence. As a result, although lacking strong evidence is usual, oncologists often have to make treatment recommendations16. This is particularly important when they make the proposal of an investigational drug use.
Saiyed et al. performed a systematic review and noted that among adult patients with cancer, 13%-71% received at least one off-label chemotherapy, mainly because of drug unapproved for specific tumour and modified drug applications. Metastatic cancers and palliative care patients received the most off-label drugs. In addition, the off-label drug use unsupported by standard treatment guidelines was in the range of 7%-31%17.
Joergeret al. performed a study including a total of 985 consecutive patients receiving 1,737 anticancer drug treatments and of them, 32.4% received at least one off-label drug. Major reasons for off-label use were the lack of approval for the specific disease entity (15.7%) and modified application of the anticancer drug (10%)18. Conti et al. examined the prevalence of off-label anticancer drug use from a population-based cohort database of medical oncologists. In this study, off-label use amounted to 30%. 14% of them were conformed to an NCCN-supported off-label indication. Total national spending on these chemotherapies amounted to $12 billion19.
According to a prior study conducted in our centre during the period 2005-200820, the majority of applications of drugs in exceptional circumstances came from Medical Oncology, Gastroenterology and Rheumatology Department. Montero et al. also reported that the majority of drugs in special situations are prescribed by oncologists (approximately 50%)21.
A study performed in 2002 in France reported that 6.7% of prescriptions presented a drug used in an off-label use. Off-label prescriptions were common in patients with hormone-refractory prostate cancer (57.6%) and in patients with bladder cancer (37.6%). The drugs most frequently used off-label were docetaxel (29%), oxaliplatin (24%), fludarabine (8%) and carboplatin (8%)22. In our centre ethiodol (58 applications), used in transcatheter arterial chemoembolization (TACE) for large hepatocellular carcinoma, oxaliplatin alone (21) and durvalumab (20) were the most requested drugs in exceptional circumstances. In addition, in our setting, drugs in exceptional circumstances were most commonly prescribed for hepatocellular carcinoma (13.4%), lung cancer (13.2%) and breast cancer (12%).
The category of some drugs included in this study has changed since the work was done. Some drugs previously considered “special uses” have been approved, reviewed and recategorized over this period, based on the increase of the level of evidence of efficacy, which nowadays is aceptable to support the use of them.
Outcomes in real-life experience
It is important to determine real-world effectiveness and toxicity of these medicines in order to avoid futile treatments in patients with a short life expectancy. Sánchez-Cuervo et al. conducted a retrospective observational study to assess the use of chemotherapy over the course of the last 30 days of life. Regarding the patients who initiated a new regimen of chemotherapy during the 30 days before their death, 35.2% of the treatments administered were drugs in special situations23.
Therefore, we studied real-world evidence about effectiveness and safety of medicines used in special situations. It is crucial to analyse its correlation to the prior available evidence. We discuss effectiveness and safety of the most frequent off-label, compassionate use and foreign drugs below. However, our study has two main limitations: the small sample size of some drugs and the presence of missing values in our data set.
For example, regarding effectiveness and safety of oxaliplatin for advanced gastric cancer, according to our results, mean age of patients was 69.4 (SD 12.3) years and the majority of them (66.7%) had an ECOG PS 1. OS was 10.5 months and 35.7% of patients experienced a disease progression. The most frequently reported adverse event was fatigue (78.5%). These results are consistent with prior studies. Kawada et al.24 reported an OS of 7.1 months (95% CI, 2.3-10.1) and in their study, the most frequently reported grade 3-4 adverse event was fatigue (20%) and hypokalemia (20%).
Palbociclib in combination with endocrine therapy is a valuable emerging option for patients with HR+/HER2− advanced or metastatic breast cancer. In Spain, palbociclib was launched in November 2017, but it was included in an on-going compassionate use programme since 2015. Some real-life studies with palbociclib in advanced breast cancer have been published. Du Rusquec et al. informed a SD, PD and PR rates of 45, 28.3, and 26.7%, respectively25. The findings of Masuda et al. include a 1-year OS and PFS of 92.9 and 75%, respectively26. In our study, at 12 months, the PFS rate was 21.2% and the median OS was 17.3 months for patients with very advanced disease, who did not meet label indications for patients with disease progression following hormone therapy. Progression disease rate was higher, 92.3%, in our study, maybe due to the characteristics of patients included (more advanced stage, a higher number of prior chemotherapy lines…), although a limitation of this work is the small sample size (n = 17).
Some studies have addressed the compassionate use of durvalumab in locally advanced and unresectable NSCLC. According to Gil-Sierraet al.27, the mean PFS was 20.8 (13.6-28.1) months and the mean OS could not be calculated because there were no deaths. They identified 17 adverse events (AEs). The most frequent AEs were: 4 (23.5%) respiratory infections, 3 (17.6%) cough and 2 (11.7%) erythematous lesions. There were 16 (94.1%) AEs grade 1, and 8 treatment interruptions were recorded, without suspensions. In our study, the mean OS was 31.3 months. The rate of grade 1 adverse effects was 60.0%. Durvalumab was discontinued in 2 patients due to adverse effects (40.0%).
Ethiodol is distributed by Guerbet (U.S.) and approved for use in over 47 countries worldwide28. A systematic review including 10,108 patients treated with ethiodol TACE have concluded that the response rate was 52.5% (CI 43.6-61.5) and overall survival (OS) was 51.8% at 2 years29. Similarly, in our study, response rate was 60%, but OS was higher, 77% at 2 years.
Our findings are consistent with those of Arroyo-Álvarez et al., who analysed the use of off-label oral antineoplastic (ANEO) drugs and oncological outcomes between 2005 and 2015. The median PFS was 5 months (4-21.3), and OS 11 months (9.2-20.6). Moreover, the most frequent reported side effects were asthenia (19%), neutropenia (10.7%), and nausea and vomiting (8.9%)30. In the same line, García-Muñoz et al. performed a retrospective study of all patients attending the Medical Oncology Department who began treatment with ANEO drugs in 2016. They compare the results obtained with the clinical evidence on which the authorisation was based. They also concluded that the effectiveness of off-label ANEO was similar to the evidence available from RCTs15. These data suggest that there are similarities in effectiveness and toxicity profiles in drugs approved for special uses.
In conclusion, a considerable number of drugs in special situations are prescribed to Oncology patients in Spanish hospitals. The majority of applications of drugs in special situations was supported by RCT results. The real-life experience showed an effectiveness and tolerance profile of drugs used in special situations similar to those described in RCTs.











 texto en
texto en