Introduction
Patient safety, health care quality and workforce development remain enduring priorities for international public health and sustainable development organizations. In 2012, IMS Institute for Healthcare Informatics estimated global costs associated with medication-related errors at USD 42 billion annually1. In the United States alone, adverse drug events accounted for an estimated USD 1.56 billion to USD 5.6 billion annual expense to hospitals and more than 770,000 injuries or deaths, according to a 2001 report by the Agency for Healthcare Research and Quality2.
In alignment with the WHO's Revised Drug Strategy on the role of pharmacist and Good Pharmacy Practice, the International Pharmacy Federation (FIP) leads the promotion of best practices and implementation of national standards which promote the advancement of the entire profession; these activities supports patient health promotion and self-care, access to medical supplies and medical devices, pharmacist-prescribing, and medication management3. By leveraging multiple stakeholders including pharmacy organizations and government agencies toward a common purpose, FIP stimulates action through the development and promotion of tools and resources at each stage of the medication use process.
Improving patient safety and health care quality initiatives requires a pharmacist workforce prepared to address the evolving current and future needs of its society. And the pharmaceutical profession is committed to preparing for these challenges4,5. FIP Development Goals6, which are consistent with the United Nations' 2030 Agenda for Sustainable Development Goals7 , support global health by enabling the advancement of pharmaceutical practice, sciences, and education6. Specifically, the FIP Development Goal 4 regarding advanced and specialist development advocates for the systematic use of professional recognition programmes, systems, and frameworks as markers for advancement and specialisation across the workforce, including advanced pharmaceutical scientists8.
Activities to advance moving global pharmacy practice from a productcentered approach to a patient-focused one must consider multiple interrelated factors from multiple levels (i.e., international, national, local, and organizational) as well as various times and places. The best possible conditions for this are the alignment of policies, regulations, political commitment, economic conditions, educational structures, adequate resources, population needs and expectations, and historical and cultural influences. However, these dynamic factors differ, and they can evolve among and within both developing and developed nations. Action plans and strategies to leverage opportunities and overcome barriers help facilitate practice advancement. Through workforce development, pharmacists at the local level in developing and developed nations cultivate transformative change that improve safety and quality of patient care.
It is challenging to contextualize the baseline of global trends and categorize patient care activities that align with advanced practice and specialized practice along the complex system. Approaches to account for variation in factors include mapping scope of practice to desired performance level, as well as role and responsibilities to knowledge, skills, and experience9.
Credentialing and privileging in health care provides an opportunity for self-regulation, peer recognition, and evaluation of a professional's education, training, experience, and competence10. The most rigorous credential to demonstrate pharmacist competency for specialist practice is board certification. Board certification serves a significant role in supporting FIP's advanced practice and specialization frameworks; it also contributes to several workforce development goals (i.e., early-career training strategy, advanced and specialist development, competency development, continuing professional development strategies)11.
Board of Pharmacy Specialties and accreditation
The Board of Pharmacy Specialties (BPS) was organized in 1976 as an independent certification agency of the American Pharmacists Association; it is located in Washington, DC, United States12. BPS is recognized as the single agency that operates across the pharmacy profession to provide specialty certification of pharmacists. By documenting the expanding and evolving role of pharmacists, it recognizes, sets standards for, and provides certification in specific clinical specialties. Most importantly, it establishes independent, objective standards that are applied in a psychometrically sound, defensible process. The overriding concern of BPS is to ensure that the public receives the level of pharmacy services that will improve a patient's quality of life.
A total of 14 specialties are currently recognized by BPS13,14, including:
Board Certified Nuclear Pharmacist (BCNP), since 1978
Board Certified Nutrition Support Pharmacist (BCNSP), since 1988
Board Certified Pharmacotherapy Specialist (BCPS), since 1988
Board Certified Psychiatric Pharmacist (BCPP), since 1994
Board Certified Oncology Pharmacist (BCOP), since 1996
Board Certified Ambulatory Care Pharmacist (BCACP), since 2009
Board Certified Critical Care Pharmacist (BCCCP), since 2013
Board Certified Pediatric Pharmacy Specialist (BCPPS), since 2013
Board Certified Geriatric Pharmacist (BCGP), since 2017
Board Certified Cardiology Pharmacist (BCCP), since 2017
Board Certified Infectious Diseases Pharmacist (BCIDP), since 2017
Board Certified Sterile Compounding Pharmacist (BCSCP), since 2018
Board Certified Transplant Pharmacist (BCTXP), since 2018
Board Certified Emergency Medicine Pharmacist (BCEMP), since 2020
BPS Board Certification trends and drivers in the U.S.
While board certification is integrated into the credentialing, privileging, and reimbursement models in medical and nursing professions, achieving board certification is generally a voluntary process in the pharmacy profession. More than 90% of physicians are board certified in the United States15, compared with about 10% of U.S. pharmacists certified by BPS. Pharmacy board certification will likely increase in the U.S. due to multiple drivers and trends. Under a collaborative agreement with physicians, pharmacists in several states (e.g., California16, Montana17, and North Carolina18) who meet specific criteria (including BPS board certification) have been granted expanded authority to prescribe medications for minor acute conditions. Furthermore, the American Society of Health-System Pharmacists (ASHP) Commission on Credentialing requires postgraduate year 2 residency program directors to be board certified if certification in the area of specialization exists19. Also, health-system employers are more likely to prefer or require pharmacist board certification for clinical pharmacy positions20, especially when organizational goals align with the value of lifelong learning and rigorously assessed recertification activities21. In addition, hospitals that use certified pharmacists perform better on process of care measures than hospitals that do not use certified pharmacists22. Finally, board certified pharmacists report personal and professional development and recognition as key factors for achieving and maintaining certification23,24.
BPS governance and administration
BPS is governed by a 12-member Board of Directors, which includes six board certified pharmacists, 3 non-specialist pharmacists, 2 non-pharmacist health professionals (e.g., physician, nurse), and a public member25.
In addition, the BPS Specialty Councils develop standards and eligibility requirements for board certification in specialty areas for approval by the BPS Board of Directors, develop examinations and set passing standards for initial and continuing certification of pharmacist specialists, and approve and review professional development programs for recertification of pharmacist specialists. A Specialty Council is composed of 9 members appointed by the BPS Board. Seven members are pharmacists holding BPS board certification in that pharmacy specialty and two members are pharmacists that do not hold board certification in the specialty area26.
Certification examination eligibility criteria
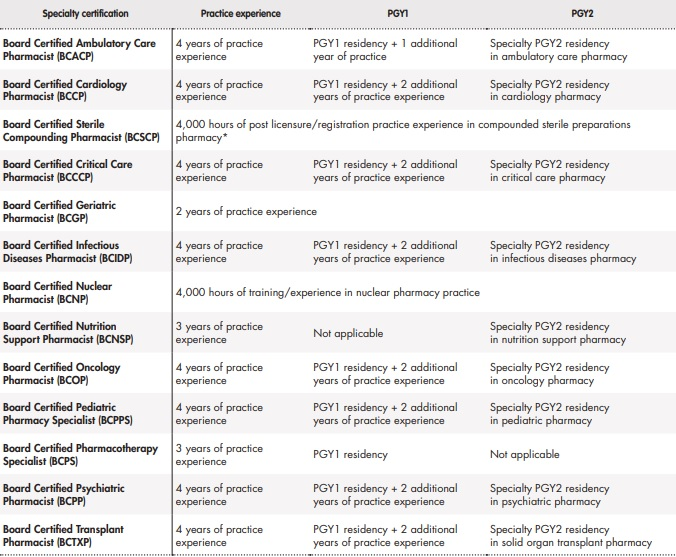
Each BPS Specialty Council is responsible for developing and determining examination eligibility for their specialty area. To be eligible, pharmacists must graduate from a pharmacy program accredited by the Accreditation Council for Pharmacy Education or a program outside the U.S. that qualified the individual to practice in that jurisdiction. Pharmacists must also have current, active license to practice pharmacy in the U.S. or another jurisdiction. All practice experience must be after licensure or registration as a pharmacist, including 50% or more of that time spent practicing in the domains described in the content outline (Table 1). Postgraduate year 1 (PGY1) and postgraduate year 2 (PGY2) pharmacy residency programs accredited by ASHP are creditable for the purpose of eligibility27.
Beginning in 2022, Canadian Pharmacy Residency Board Accredited year 1 pharmacy residency programs are recognized as an approved standard for meeting BPS requirements for eligibility pathways that recognize PGY1 residency programs. Pharmacists completing residencies that are not ASHP-accredited under the current BPS policy are still eligible to apply under the practice experience pathway. Furthermore, BPS stated that residences accredited under the ASHP International Standard meet eligibility pathways that recognize PGY1 residency programs28.
All BPS certification examinations have 175 items and use a 4-option multiple-choice format with some alternate item types (e.g., multiple-correct response, drag and drop, image and video supplementation).BPS administers certification examinations twice a year during an April-May test window and a September-October test window27.
Beginning in 2023, revisions to the eligibility criteria for the Board Certified Ambulatory Care Pharmacist (BCACP) and Board Certified Geriatric Pharmacist (BCGP) certification examinations will be implemented (Table 2). The revisions were approved by BPS to promote better alignment among eligibility pathways across all BPS specialties and recognizes the value of residency training while maintaining flexible eligibility pathway options for candidates29.
Table 2. BPS certification examination practice experience eligibility criteria revisions beginning January 1, 202319

Maintenance of certification
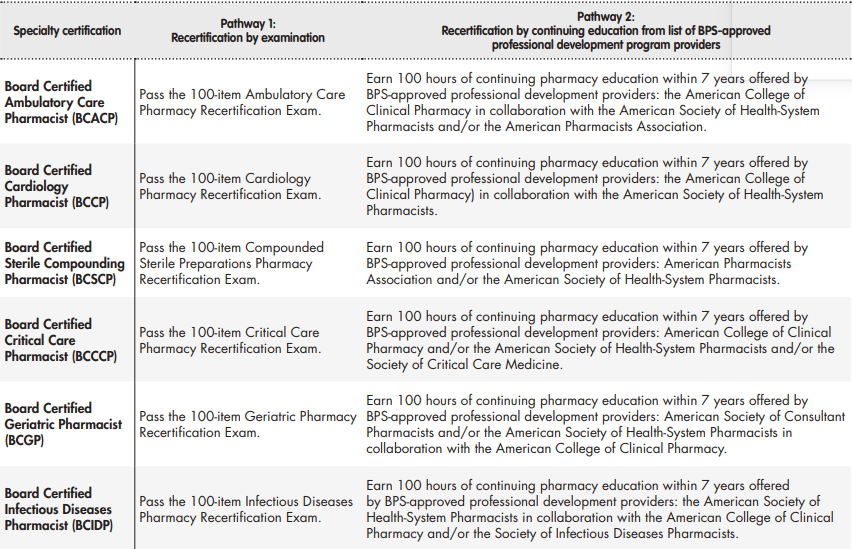
Each BPS Specialty Council is responsible for determining the requirements for maintaining certification in a specialty area. Board certified pharmacists must maintain current and active license to practice pharmacy in the U.S. or another jurisdiction. A certification cycle is 7 years in duration, and board certified pharmacist must either earn a prescribed number of continuing pharmacy education (CPE) credits offered by a BPS-approved professional development program or successfully achieve a passing score on the 100-item recertification examination administered by BPS (Table 3)30.
Table 3. (cont.). BPS recertification requirements as of July 2021
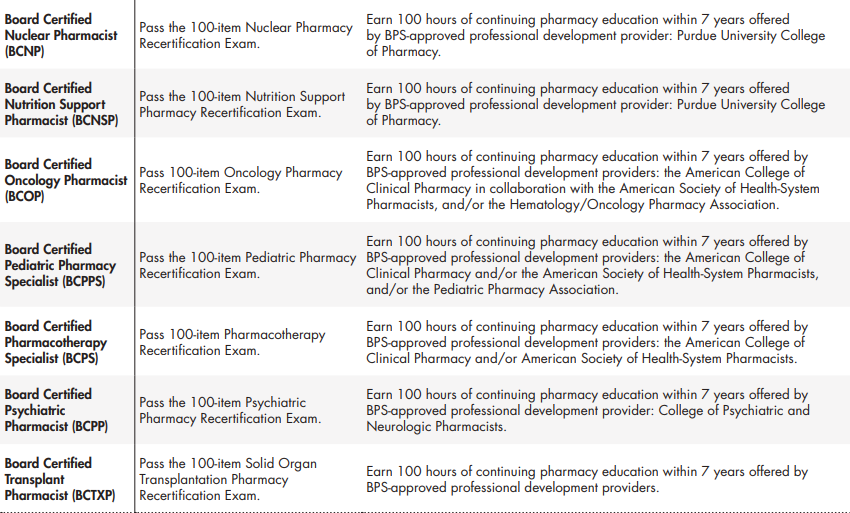
*The initial cohort of certificants in new specialty areas will be eligible to recertify by exami-nation in year 7 of the certification cycle (i.e., BCCCP and BCPPS in 2022, BCCP and BCIDP in 2025, BCSCP in 2026, BCTXP in 2028).
As of July 2021, over 51,500 active pharmacist board certifications are issued by BPS in U.S. and abroad. (Figure 1), including more than 5,600 active certifications outside the U.S. (Figure 2)12. Pain Management Pharmacy is being explored under the current process as a potential specialty.
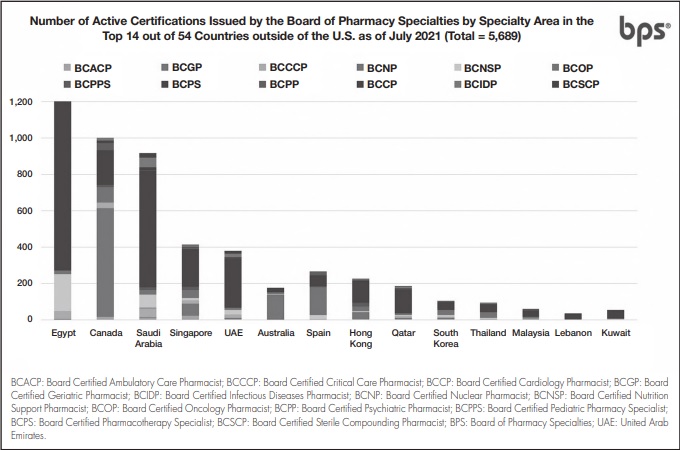
Figure 2. Number of active certifications issued by BPS by specialty area in the top 14 out of 54 countries outside of the U.S.12
Accreditation provides validation that certification programs adhere to competency assurance standards to the public and other stakeholders. BPS currently has nine programs accredited by the National Commission on Certifying Agencies (NCCA): Nuclear Pharmacy, Nutrition Support Pharmacy, Pharmacotherapy, Psychiatric Pharmacy, Oncology Pharmacy, Ambulatory Care Pharmacy, Critical Care Pharmacy, Pediatric Pharmacy, and Geriatric Pharmacy12. BPS plans to submit new certification programs for NCCA accreditation after meeting the standards and application eligibility requirement thresholds, which include a minimum of one year of examination administration of the assessment or a minimum of 500 candidates assessed. NCCA accreditation is valid for a period of 5 years31 BPS is also accredited by the International Accreditation Service under the International Organization for Standards (ISO) 17024 standard12.
Board certification intersects with advance practice and specialization frameworks, and it is driven by multiple factors including workforce development, credentialing and privileging, remuneration and payment models, and government mandates at the local levels.
Professional recognition, credentialing and privileging of advanced pharmacy practice
Pharmacists working at a recognized level of advancement, with higher sets of competencies, improve and safeguard patient safety and more effectively manage complexity in many areas of expert practice. Professional recognition of advanced practice improves acceptance by other colleagues not only in the clinical team, but also in other areas of practice such as research, education, and management. Recognizing pharmacists as advanced practitioners enables employers and senior managers to have evidence of capability32. Recognition of specialty practice is extremely variable among different countries, both in the pathways followed and the responsible organization. However, different states in the U.S. and countries around the world that have some recognition of advanced pharmacy practice recognize the certifications from the BPS10.
PharmD curriculum and FIP Framework in Thailand
In 2020, Thai pharmacists published findings from its qualitative research based on a modified FIP framework, including a competency framework that was developed to identify opportunities for improvement and prepare a workforce that the serves all aspects along the pharmaceutical supply chain and care delivery continuum. Since 2014, the 6-year Doctor of Pharmacy degree transitioned from generalist to specialist pharmacist competencies33. Like many counties, ongoing interest in advanced skills in pharmacy practice persists12.
Medication management review accreditation and renumeration in Australia
Australian pharmacists receive renumeration as part of a governmentfunded medication management review program through the Society of Hospital Pharmacists of Australia's (SHPA) accreditation program. In order to become eligible for SHPA accreditation, a pharmacist must successfully earn BCGP or BCPS from BPS or earned credentialing from the National Alliance for Pharmacy Education34.
Government mandate in Egypt
The Egyptian Ministry of Health mandated that all hospitals must implement clinical pharmacy practices before July 201335. The following year, a mandate was issued to increase pharmaceutical education from 5 to 6 years to accommodate hospital, community, and industry training experiences36. Significant interest in board certification remains in Egypt.
Hospital pharmacy in Spain
As part of its strategic plan to implement a specialization program in Spain, the Spanish Society of Hospital Pharmacists set a goal to integrate board-certified pharmacists within its hospitals and health systems37. This would increase the professional competence of hospital pharmacy specialists and support a legal requirement for a recognition of subspecialty areas of hospital pharmacy practice. The total population of board certified pharmacists in Spain increased by 220% between 2009 and 2021 (Tabla 4)38. In addition, Spanish pharmacists lead in the number of active certifications issued in Europe (Table 5) and number of Board Certified Oncology Pharmacists (BCOP) issued outside of the United States (Table 6)38.
Table 4. Active certifications issued in Spain September 2009 compared to September 202138

BCACP: Board Certified Ambulatory Care Pharmacist; BCCCP: Board Certified Critical Care Pharmacist; BCGP: Board Certified Geriatric Pharmacist; BCIDP: Board Certified Infectious Diseases Pharmacist; BCNSP: Board Certified Nutrition Support Pharmacist; BCOP: Board Certified Oncology Pharmacist; BCPP: Board Certified Psychiatric Pharmacist; BCPPS: Board Certified Pediatric Pharmacy Specialist; BCPS: Board Certified Pharmacotherapy Specialist.
Table 6. Active BCOP certifications issued in the U.S. and abroad as of September 202138

BCOP: Board Certified Oncology Pharmacist.
Due to its recognition by pharmacy managers and other healthcare professionals, BPS certification is an explicit qualification in several job openings for hospital pharmacists, particularly in onco-haematology hospital pharmacy practice. As in other countries, it is envisioned that in the years to come, certifications from independent organizations of acknowledged reputation in a certain professional area may be incorporated as a curricular merit in recruitment competitions.
Conclusion
Pharmacists have a lifelong commitment to maintain and improve professional knowledge and competence as well as to apply knowledge, experience, and skills to assure optimal patient outcomes. Board certification is way approach the profession demonstrates its ongoing commitment to patient safety, health care quality, and workforce development as it meets the evolving needs of society. A rigorous, objective, standard such as board certification should be integrated into frameworks and programs that advance practice and specialization. In this regard, over the last few years BPS has become recognized international certifying agent in pharmacy specialty programs in the United States and in many countries around the world.
Spain leads in the number of pharmacist board certifications in Europe and serves as an international reference for the number of pharmacists who hold board certification in oncology pharmacy. The growth in new specialty areas as well as the number of international board certification demonstrate significant interest and extraordinary effort among pharmacists in Spain and other counties who are focused on professional development in specific clinical areas with specialization. With increasing awareness among hospital pharmacists in Spain, we foresee board certification as an element for improving pharmaceutical care and patient care.











 texto en
texto en