Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de la Sociedad Española del Dolor
versión impresa ISSN 1134-8046
Rev. Soc. Esp. Dolor vol.23 no.1 Madrid ene./feb. 2016
ORIGINAL
Inter- and intra-patient variability in breakthrough pain episodes of opioid-treated patients with underlying chronic pain. An observational, prospective and multicenter study
Variabilidad inter- e intra-individual en pacientes con dolor irruptor tratados con opioides. Estudio observacional, prospectivo y multicéntrico
J. Pérez Cajaraville, L. Cánovas1, J. Santos2, E. Ortega3, J.J. Cuello4, R. Alborés5, J.A. Sáenz6, F.J. Vara7, J. Carceller8, J. Sobrino9, M. Carpintero10, A. Gutiérrez11, C. Cabanas12 and E. Torcal13
Unidad Funcional Tratamiento del Dolor. Grupo HM Hospitales. Madrid
1Complejo Hospitalario de Ourense.
2Hospital Clínico Universitario de Salamanca.
3Hospital Río Hortega. Valladolid.
4Complejo Asistencial de Zamora.
5Hospital Comarcal de Monforte de Lemos. Lugo.
6Hospital San Pedro. Logroño.
7Pain Unit and Palliative Care. Hospital Universitario de Salamanca.
8Pain Unit. Hospital Clínico Universitario. Santiago de Compostela.
9Hospital de Povisa. Vigo.
10Hospital de Cabueñes. Gijón.
11Hospital Universitario de Burgos.
12Complejo Universitario Xeral-Cíes. Vigo.
13Medical Department. Ferrer Farma. Grupo Ferrer. Barcelona. Spain
ABSTRACT
Objective: Since, to date, there are few epidemiological data assessing the diversity in the characteristics of breakthrough pain episodes, the present study was performed to assess the intra-individual variability in the episodes of breakthrough pain in patients with underlying chronic pain controlled with opioids.
Methods: An observational, prospective and multicenter study (CADI study) was conducted in the context of the routine clinical practice of Spanish pain specialists recruiting opioid-treated patients with underlying chronic pain. Data were recorded in three visits (baseline, at 7 and 28 days post-inclusion) and by the patient on a patient's diary card, specifically designed to characterise the first 8 breakthrough pain episodes (type, intensity -using 100 mm Visual Analog Scale- and duration of pain), to assess the intra-individual and inter-individual variability in the intensity, duration and typology of episodes of breakthrough pain.
Results: 50 opioid-treated patients were recruited (23 with oncologic pain and 27 with non oncologic pain, mean age of 61.1 years, 62 % females). For all three parameters, inter-patient variability was higher than intra-patient variability throughout the episodes. Nevertheless, we found intra-patient variability in maximum pain intensity, pain intensity at the end of the episode, pain relief and duration of the episode.
Conclusions: This is the first study to quantify the intra-patient variability of breakthrough pain. The results show a great variability in terms of intensity and duration of the episode and its typology. Although inter-patient variability is higher, the intra-patient variability is important enough to be taken into account in optimizing the approach and treatment selection.
Key words: Breakthrough pain, chronic pain, opioids, oral transmucosal fentanyl, variability.
RESUMEN
Objetivos: Debido a los pocos datos epidemiológicos existentes que evalúen la diversidad de las características de los episodios de dolor irruptivo, se realizó el presente estudio, cuyo principal objetivo fue evaluar la variabilidad intraindividual de las crisis de dolor irruptivo en pacientes con dolor crónico controlado con opioides.
Métodos: Este estudio observacional, prospectivo y multicéntrico (estudio CADI) se llevó a cabo en el contexto de la práctica clínica habitual, en Unidades del Dolor de España, con la participación de pacientes tratados con opioides para el dolor crónico. Los datos fueron registrados en tres visitas (basal, a los 7 y 28 días después de la inclusión) y por el propio paciente, en un Diario del Paciente, específicamente diseñado para caracterizar los primeros 8 episodios de dolor irruptivo (tipo, intensidad -utilizando la Escala Analógica Visual (EVA)- y duración del dolor) con el objetivo de evaluar la variabilidad intraindividual e interindividual en la intensidad, duración y tipología de los episodios de dolor irruptivo.
Resultados: Se reclutaron 50 pacientes, 23 con dolor oncológico y 27 con el dolor no oncológico (edad media de 61,1 años; 62 % de mujeres). Aunque para los tres parámetros medidos, la variabilidad entre pacientes fue mayor que la variabilidad intrapaciente, la variabilidad intraindividual fue significativa en la evaluación de la máxima intensidad del dolor, la intensidad del dolor al final del episodio, el alivio del dolor y la duración del episodio de dolor irruptivo.
Conclusiones: Este es el primer estudio que cuantifica la variabilidad intraindividual del dolor irruptivo. Los resultados muestran una gran variabilidad en cuanto a la intensidad y la duración del episodio y su tipología. Aunque la variabilidad entre pacientes es mayor, la variabilidad intrapaciente es lo suficientemente importante como para ser tenida en cuenta para la mejor aproximación y selección del tratamiento.
Palabras clave: Dolor irruptivo, dolor crónico, opioides, citrato fentanilo oral transmucosa, variabilidad.
Introduction
Pain management is a fundamental human right recognized by the United Nations and the World Health Organization (WHO) (1-4). Pain, especially chronic pain, is a key patient-reported outcome. Pain poor control undermines quality of life (1) and, as stated by Dr. Milton Raff, "its physical, psychological, social, and economic ramifications evolve, overlap, and compound one another" (1,5). Effective treatment of chronic pain improves the overall quality of life, including maintenance of function and interaction with family and friends (1,6).
Currently, despite the increasingly sophisticated understanding of the pathophysiology of pain, widespread inadequacy of its treatment is still a reality (1,7). The lack of knowledge on the characteristics of the different types of pain (as cancer or non cancer pain or breakthrough pain) is considered as one of the barriers for correct pain management (7-9).
Episodes of breakthrough pain, defined as a transitory exacerbation of pain experienced by the patient who has relatively stable and adequately controlled baseline pain (10-12), are an important contributor to suffering in these patients (13,14), occurring in 33-65 % of patients with chronic cancer pain and in 70 % of patients with chronic non cancer pain (12).
In the absence of knowledge on the characteristics of this type of pain, particularly regarding its management and its differences depending on the baseline pathology (cancer or non cancer patients) (10,11), recent observational studies have addressed this issue (15,16), pointing out that breakthrough cancer pain is an extremely heterogeneous condition.
In this context, the present observational, prospective and multicenter and nationwide study (CADI study) was conducted in the routine clinical practice to assess the inter- and intra-individual variability in the intensity, duration and typology of episodes of breakthrough pain in patients with underlying chronic pain controlled with opioids.
Results obtained would contribute to a better knowledge of this type of pain and, thus, improving pain management in routine clinical practice.
Methods
From Dec 2011 to Sep 2012, an observational, prospective and multicenter and nationwide study (CADI study) was conducted in the context of the routine clinical practice of 20 Spanish specialists from pain units. Each investigator consecutively enrolled 4 opioid-treated patients with underlying chronic pain (two patients with cancer pain and two patients with non cancer pain), to complete a study sample of 56 patients.
The included patients had to be older than 18 years, receive treatment with opioids for their chronic pain in an outpatient basis without variations in the regimen for treatment of chronic pain during the last 4 weeks, with a maximum of 3 episodes of breakthrough pain per day and be treated in pain units from different Spanish regions. Dose titration for the treatment of breakthrough pain had to be previously established and the same dose had to be used for the last 4 episodes before the start of the study. Exclusion criteria for the study included: being hospitalized during the last month due to uncontrolled pain or surgery, undergoing radiotherapy in the last month or having a scheduled radiotherapy session within the first month of the study, having a Karnofsky index ≤ 50, being terminally ill (life expectancy < 15 days), being pregnant or at risk of pregnancy (without taking adequate contraceptive measures) and being not capable to understand the objectives of the study and completing the questionnaires.
The study protocol was approved by the Ethics Committee of Human Experimentation of Clínica Universidad de Navarra (Pamplona, Spain) and procedures were in accordance with the ethical standards laid down in Helsinki Declaration, as revised in 2000.
Prior to participation, patients provided written informed consent. During the basal visit, the physician collected demographic, clinical (including comorbidities) and treatment (for the underlying chronic pain, for breakthrough pain and for comorbidities) data from the patient in the investigator e-CRF (Case Report Form) specially designed for this purpose. Basal functional status was assessed by means of Karnofsky Performance Status Scale (17) and basal underlying chronic pain by means of the 100-mm Visual Analog Scale (VAS) (18).
At the end of the basal visit, the physician provided patient with the patient's diary card, specifically designed to characterise the first 8 breakthrough pain episodes (type, intensity and duration of pain) by the patient. Type was characterized according to the nature (superficial, oppressive, deep, burning, stinging, electric, tingling or unknown) and onset of pain (sudden or gradual); intensity was characterized according to maximum pain intensity during and at the end of the episode. Moreover, patients were asked to characterize their pain as incidental ("episode associated with something") or spontaneous ("episode without apparent cause").
Patients were asked to not change the treatment regimen (drug, dose) during the first 8 episodes. In case of unbearable pain, patients had to contact the study investigator, who valued the convenience to change the established regimen and all changes were recorded in the eCRD.
Episodes during which treatment changes were performed due to unbearable pain were not considered for the primary analysis. Intensity of breakthrough pain episodes was assessed by means of the 100-mm Visual Analog Scale (VAS) (18).
A follow-up visit was performed 7 ± 2 days after patient's inclusion to report current treatment for basal pain and for breakthrough pain and to collect the first part of the diary card. In the final visit, performed 28 ± 2 days after patient's inclusion, functional status (Karnofsky Performance Status Scale) and pain (VAS) were assessed again and information on current treatment for basal and breakthrough pain was also collected, together with the second part of the patient's diary card.
The primary objective was to assess the intra-patient and inter-patientvariability in the intensity, duration and typology of episodes of breakthrough pain in patients with underlying chronic pain controlled with opioids. The diagnosis of chronic pain was established before the beginning of the study according to routine clinical criteria. Types of chronic pain in terms of pathophysiology were recorded (nociceptive, neuropathic or mixed).
Secondary objectives included description of chronic pain characteristics, etiology, treatment (currently and within the last month before inclusion), registry of the number of episodes during the study period and description of prescribed treatments for episodes of breakthrough pain, time between administration of treatment of chronic pain and the start of episodes of breakthrough pain, patient's self-reported treatment for episodes of breakthrough pain (excellent, good, regular, ineffective).
Since the main objective evaluated intra-individual variability in the intensity, duration and type of breakthrough pain, it was considered necessary to have at least three of the first eight episodes reported into the patient's diary card, to assess this variability, and declared invalid patients with less than three episodes documented.
Study variables were obtained from the patient's self-reported registries on the episodes and from the patient's data registered by the investigator in the e-CRF.
Statistical analysis
Results were expressed as measures of central tendency and dispersion (mean and standard deviation) for continuous variables and as absolute numbers and relative frequencies (%) for categorical variables. Comparisons in the clinical variables between subgroups of subjects (obtained a posteriori) were conducted using a Student's t-test for continuous variables and Pearson's chi-squared test or Fisher exact test for categorical variables. In order to find possible differences, results were expressed for cancer and non cancer patients as well as for the total of them. Variability of intensity of pain, pain relief and duration of pain episodes were analyzed using the repeated measurements analysis of variance. In all cases the variable distribution was checked against theoretical models and the hypothesis of variance homogeneity was corroborated. Statistical significance was set at a p-value of p < 0.05. The statistical analysis was performed using SAS v.9.2 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Fifty-six patients were recruited for this study, 6 (10.7 %) of whom were excluded because less than 3 episodes of breakthrough pain were well documented. Therefore, the intra-individual variability of the intensity, duration and type of breakthrough pain episodes were assessed in the remaining 50 (89.3 %) eligible chronic pain patients, 23 experiencing cancer pain and 27 non cancer pain.
Mean (SD) age of patients was 61.1 (14.6), age ranging from 33.5 to 89.6 years; 90 % of patients were Caucasian, and 62 % were female. More prevalent co-morbidities at baseline were arterial hypertension (54 %), osteoarthritis (34 %), dyslipidaemia (30 %) and gastroesophageal reflux disease (18 %); other disorders such as diabetes mellitus, ischemic heart disease, renal failure or liver diseases were less frequent (< 12 %).
Demographic data distributed according to the oncologic and non oncologic type of pain are shown in Table I. No relevant differences were observed in the functional status scores (Karnofsky index) in both groups at baseline or during the study.
Mean (SD) pain intensity was 48.5 (30.6) mm at baseline, 42.7 (25.6) mm after 7 days and 37.4 (23.0) mm after 30 days, without differences between both cancer and non-cancer patients at any time-point.
A total of 389 episodes of breakthrough pain were documented during the study, 88 of which were discarded because the rescue treatment was not described (36 episodes) or because the rescue treatment was different than that used in the previous 4 weeks (52 episodes); thus, the remaining 301 episodes, in which the rescue treatment was the same during 8 weeks, were used for the intra-individual variability analysis of breakthrough pain.
During the last month before the inclusion in the study, the most frequent treatments for chronic cancer pain were fentanyl (19.6 %), paracetamol (7.8 %) and either duloxetine or ibuprofen or pregabalin or tramadol (5.8 %, each), whereas for patients with non cancer pain fentanyl (13.7 %), pregabalin (12.3 %) and gabapentin (9.5 %) were the most frequently used treatments.
During the study, the most frequent treatments for underlying chronic cancer pain were fentanyl (26.0 %), oxycodone (13.0 %), followed by either etoricoxib or ibuprofen or metamizole or tramadol (8.6 % each), whereas for patients with non cancer pain most frequent treatments were gabapentin (14.8 %) followed by either duloxetine or fentanyl or ibuprofen or oxycodone or pregabalin (7.4 %, each) (Table II).
The most frequent treatments for the last four breakthrough pain episodes before the inclusion in the study were fentanyl (55.5 %) and paracetamol (18.5 %) in cancer pain patients, and fentanyl (77.1 %) and paracetamol (8.5 %) in non cancer patients. In both types of patients fentanyl was orally and nasally administered by transmucosal routes.
Fentanyl was the most frequently used rescue treatment during the first 8 breakthrough pain episodes, administered alone (72.2 % of episodes) or in combination with other drugs such as baclofen (1 episode), paracetamol (4 episodes), paracetamol plus metamizole (1 episode) or tramadol (1 episode). The association of tramadol plus paracetamol was the second most frequent treatment (5.7 %) and morphine (4.5 %), the third.
No differences were found in the number of breakthrough pain episodes (Wilcoxon test: Z = 0.70, p = 0.483) or in the number of breakthrough pain episodes per week (Wilcoxon test: Z = 1.64, p = 0.100) between the cancer and noncancer patients (Figure 1).
Breakthrough pain onset was gradual in the 61.7 % of pain episodes and sudden in the remaining 38.3 %, spontaneously starting in 73 % of episodes or with a triggering factor in the remaining 27 % of cases. Pain was described as burning (29.9 %), superficial (5 %), oppressive (57.1 %), squeezing (22.3 %), sharp (46.5 %), crampy (22.9 %), tingling (10.0 %) and non- classified (0.7 %).
When fentanyl was used as rescue treatment, mean (SE) pain relief was 49.4 (20.9) mm and median duration of pain was 30 minutes. When fentanyl was combined with either baclofen or paracetamol, the duration of pain was reduced to 10 minutes (Tables III and IV). When other rescue treatments were tramadol plus paracetamol combination (5.7 % of episodes) followed by morphine (4.5 % of episodes), the mean (SE) pain relief was 42.4 (18.4) and 40.8 (13.3), respectively, and the median (IQR) duration of pain to relief was 109.5 (50-600) minutes and 360 (90-765) minutes, respectively.
Mean (SE) time from taking the last medication for chronic pain to the start of breaktrhough pain episode was 6.9 (5.5) hours; 6.7 (5.8) hours in oncologic pain patients and 7.0 (5.3) in non oncologic patients.
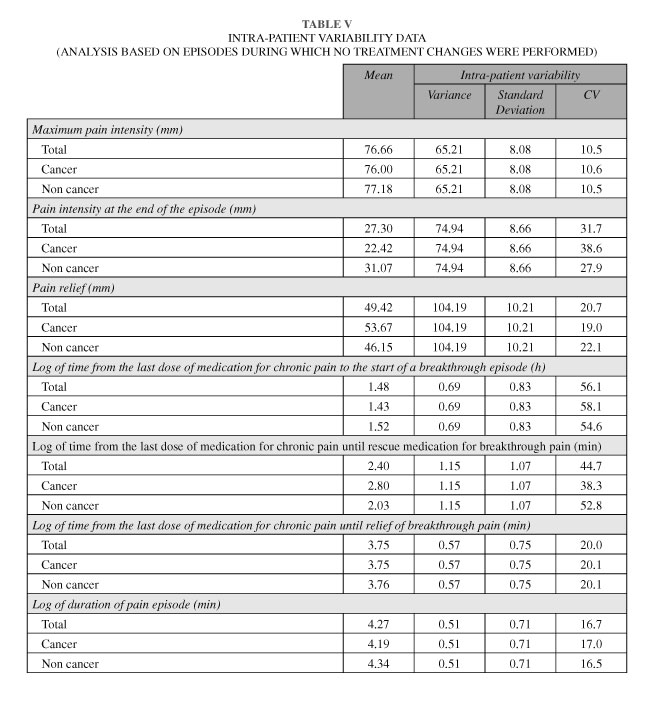
Regarding satisfaction with treatment, from a total of 253 episodes treated with fentanyl, treatment was considered as excellent in 48 (19.0 %) episodes and good in 151 (59.7 %), regular in 53 (20.9 %) and inefficacious in one episode (0.4 %) Maximum pain intensity during the episodes recorded by the patients ranged from 35 to 100 mm, with a mean (SD) of 76.7 (14.3) mm, without differences between oncologic and non oncologic patients (F = 0.25, p = 0.62). Inter-patient variability of intensity pain was higher than the intra-patient variability in the intensity pain records (variances: 146.8 and 65.2, respectively) (Figure 2.A). Nevertheless, the intra-patient confidence interval (95 % CI) of the mean for maximum pain intensity (76.66 ± 15.84) showed that the same patient could have experienced both episodes of moderate pain (≤70) and episodes of severe pain (> 70) (Table V).
Pain relief (defined as maximum pain intensity minus final pain intensity at the end of the episode) ranged from -10 to 100 mm, with a mean (SD) in total episodes of 49.4 (19.7). Inter-patient variability of pain relief was also higher compared to the intra-patient variability in the relief pain records (variances: 265.8 and 104.2, respectively) (Figure 2.B). Although a slight trend to higher values in the pain relief assessment was observed for patients with cancer pain, no statistically significant differences were found (F = 1.96, p = 0.163). As in the case of maximum pain intensity, we also found data indicative of intra-patient variability, with an intra-patient confidence interval (95 % CI) of the mean pain relief (49.22 ± 20.01) showing that in the same patient, pain relief can range between 30 and 70 points (Table V). Similarly, we also found intra-patient variability (27.30 ± 16.97) in the pain intensity at the end of the episode, thus showing that pain could range from 10 to 40 at the end of an episode for a same patient (Table V).
Pain duration of episodes ranged from 0 to 1,335 minutes (approximately 23 hours), with a median (IQR) of 60 (35, 120) minutes, without statistically significant differences being found between oncologic pain and non oncologic pain (F = 1.23, p = 0.27). Again, inter-patient variability of pain duration was higher compared to the intra-patient variability (variances: 0.54 and 0.51, respectively, time data transformed in logarithm) (Figure 2.C). However, intra patient variability was also high [median (CI): 71.5 (17.5-295) minutes] indicating that in the same patient, an episode can last either a few minutes or a few hours (Table V).
We also found intra-patient variability in the time from the last dose of medication for chronic pain to the start of a breakthrough episode, until rescue medication for breakthrough pain and until relief of breakthrough pain (Table V).
While no statistically significant differences were observed in either group in terms of pain location (chi-square = 5.64; p = 0.245), pathophysiology of pain was not homogenous between groups (chi-square = 12.8; p = 0.003). Mixed pathophysiology (nociceptive and neuropathic) (52 %) and nociceptive pain (21.7 %, somatic or visceral) were predominant in the group of patients with oncologic pain, whereas neuropathic pain (48.1 %) was predominant in the group of patients with non oncologic pain (Table I).
Finally, no serious adverse events were recorded during the study. One patient with cancer pain experienced excessive somnolence and one patient with non cancer pain experienced nausea and vomiting.
Discussion
To date, few data exist on intra-patient variability of the breakthrough pain episodes. In this regard, for the first time, results of our present observational and prospective study have provided new insights into the diversity of breakthrough pain episodes in both cancer and non cancer patients, particularly in terms of intra-patient variability. Taken together, in the presence of relevant inter-patient variability (15,16), non-negligible intra-patient diversity have been observed in our study, especially in terms of pain intensity, pain relief and duration.
If we translate these findings to clinical practice, physicians can encounter a great difficulty in establishing the most appropriate treatment regimen for each specific patient and for all his/her episodes. The first difficulty is related to the effective dose: our results reveal that in a same patient, a presumed effective dose, searched in an individual dose titration process, can not be equally effective in all episodes of the patient, due to the high diversity of them. Likewise, the variability observed in the pain intensity at the end of the episode in the same patient implies that the same treatment can not be always appropriate for all episodes. Similarly, as the duration of the episode is highly variable, the same dose can not be equally effective in all cases. This variability is also reflected in the profile of the episodes onset, with approximately 60 % gradual type and 40 % sudden type. It is very important to note that this high intra-patient variability has been observed in a maximum of 8 consecutive episodes of breakthrough pain in a same patient.
Second, another difficulty is choosing the most appropriate treatment. Although currently rapid-onset opioids are accepted as the appropriate treatment to relieve breakthrough pain (12,19,20), the variability observed in our study suggests that only those formulations that provide flexibility in dosing would be the treatment of choice for this type of pain (12,19,20). Different rapid-onset opioids' (ROOs) technologies have been developed to provide fast pain relief with potent opioid drugs such fentanyl, delivered by non-invasive routes, including oral transmucosal fentanyl citrate (OTFC), fentanyl buccal tablet, sublingual fentanyl, intranasal fentanyl spray, fentanyl-pectine nasal spray and fentanyl buccal soluble film, which have shown better efficacy than placebo or oral opioids. However, OTFC is the only product of this new generation of delivery systems that could offer this flexibility in dosing (20). The fact that this formulation is on a handle may enable to use the drug as needed, depending on the characteristics of the pain episode. It means that if excessive drug effect is produced or enough relief, the remaining dose can be removed from the mouth (19-22). Nevertheless, further studies with OTFC in breakthrough pain are necessary to assess this flexibility.
Even considering that the number of episodes monitored is important enough, due to the relatively low number of patients included in our study, and based on the high variability observed, future research should be performed to assess the convenience of dose titration, a process that is already currently being questioned when rapid-onset opioids are prescribed (20), and to confirm the high diversity observed in the breakthrough pain episodes, especially in terms of intra-patient variability.
Moreover, the study reflect the experience of a number of patients who were receiving care from pain team specialists that may not be entirely representative of a wider population. Further research should also focus on the individual source of variation, thus allowing obtaining patient profiles with specific pain characteristics and treatment preferences, as proposed elsewhere (23,24).
Although it is accepted that breakthrough pain is a heterogeneous condition and the episodes vary both between individuals and within individuals, to date no other studies had quantified the intra-patient variability of breakthrough pain.
Overall, we can conclude that the results show a great variability in terms of intensity and duration of the episode and its typology. Although inter-patient variability is higher, the intra-patient variability is important enough to be taken into account in optimizing the approach and treatment selection.
References
1. Brennan F, Carr DB, Cousins M. Pain management: A fundamental human right. Anesth Analg 2007;105:205-21. doi: 10.1213/01.ane.0000268145.52345.55. [ Links ]
2. Fishman SM. Recognizing pain management as a human right: A first step. Anesth Analg 2007;105:8-9. doi: 10.1213/01.ane.0000267526.37663.41. [ Links ]
3. Anwari JS. Pain management: Citizenship or human right? Anesth Analg 2008;106:678. [ Links ]
4. Yates JW, Kirch R. Regulatory barriers for adequate pain control. Asian Pac J Canver Prev 2010;11:17-21. [ Links ]
5. Ospina MB, Taenzer P, Rashiq S, MacDermid JC, Carr E, Chojecki D, et al. A systematic review of the effectiveness of knowledge translation interventions for chronic noncancer painmanagement. Pain Res Manag 2013;18:e129-41. [ Links ]
6. Ehrich EW, Bolognese JA, Watson DJ, Kong SX. Effect of rofecoxib on measures of health-related quality of life in patients with osteoarthritis. Am J Manag Care 2001;7:609-16. [ Links ]
7. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287-333. doi: 10.1016/j.ejpain.2005.06.009. [ Links ]
8. Ogboli-Nwasor E, Makama J, Yusufu L. Evaluation of knowledge of cancer pain management among medical practitioners in a low-resource setting. J Pain Res 2013;6:71-77. doi: 10.2147/JPR.S38588. [ Links ]
9. Zeppetella G, O'Doherty CA, Collins S. Prevalence and characteristics of breakthrough pain in patients with non-malignant terminal disease admitted to a hospice. Palliat Med 2001;15:243-6. doi: 10.1191/026921601678576220. [ Links ]
10. Portenoy RK, Bennett DS, Rauck R, Simon S, Taylor D, Brennan M, et al. Prevalence and characteristics of breakthrough pain in opioid-treated patients with chronic non cancer pain. J Pain 2006;7:583-91. doi: 10.1016/j.jpain.2006.02.003. [ Links ]
11. Davies AN, Vriens J, Kennett A, McTaggart M. An observational study of oncology patients' utilization of breakthrough pain medication. J Pain Symptom Manage 2008;35:406-11. doi: 10.1016/j.jpainsymman.2007.05.010. [ Links ]
12. Smith H. A comprehensive review of rapid-onset opioids for breakthrough pain. CNS Drugs 2012;26:509-35. doi: 10.2165/11630580-000000000-00000. [ Links ]
13. Fine PG, Busch MA. Characterization of breakthrough pain by hospice patients and their caregivers. J Pain Symptom Manage 1998;16:179-83. doi: 10.1016/S0885-3924(98)00045-1. [ Links ]
14. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: Characteristics and impact in patients with cancer pain. Pain 1999;81:129-34. doi: 10.1016/S0304-3959(99)00006-8. [ Links ]
15. Davies A, Buchanan A, Zeppetella G, Porta-Sales J, Likar R, Weismayr W, et al. Breakthrough cancer pain: An observational study of 1000 European oncology patients. J Pain Symptom Manage 2013;46:619-28. doi: 10.1016/j.jpainsymman.2012.12.009. [ Links ]
16. Davies A, Zeppetella G, Andersen S, Damkier A, Vejlgaard T, Nauck F, et al. Multi-centre European study of breakthrough cancer pain: Pain characteristics and patient perceptions of current and potential management strategies. Eur J Pain 2011;15:756-63. doi: 10.1016/j.ejpain.2010.12.004. [ Links ]
17. Friendlander AH, Ettinger RL. Karnofsky performance status scale. Spec Care Dentist 2009;29:147-8. doi: 10.1111/j.1754-4505.2009.00088.x. [ Links ]
18. Huskisson EC, Jones J, Scott PJ. Application of visual-analogue scales to the measurement of functional capacity. Rheumatol Rehabil 1976;15:185-7. doi: 10.1093/rheumatology/15.3.185. [ Links ]
19. Mercadante S, Villari P, Ferrera P, Casuccio A, Mangione S, Intravaia G. Transmucosal fentanyl vs. intravenous morphine in doses proportional to basal opioid regimen for episodic-breakthrough pain. Br J Cancer 2007;96:1828-33. doi: 10.1038/sj.bjc.6603811. [ Links ]
20. Mercadante S. Oral trasmucosal fentanyl citrate for breakthrough pain treatment in cancer patients. Expert Opin Pharmacother 2012;13:873-8. doi: 10.1517/14656566.2012.663353. [ Links ]
21. Egan TD, Sharma A, Ashburn MA, Kievit J, Pace NL, Streisand JB. Multiple dose pharmacokinetics of oral transmucosal fentanyl citrate in healthy volunteers. Anesthesiology 2000;92:665-73. doi: 10.1097/00000542-200003000-00009. [ Links ]
22. Porta-Sales J, Garzón C, Julià J, Casals M. Dolor irruptivo en cáncer. Med Clin (Barc) 2010; 135(6): 280-5. doi: 10.1016/j.medcli.2010.02.008. [ Links ]
23. Fornasari D. Pain mechanisms in patients with chronic pain. Clin Drug Investig 2012;32:45-52. doi: 10.2165/11630070-000000000-00000. [ Links ]
24. Ablin JN, Buskila D. Personalized treatment of pain. Curr Rheumatol Rep 2013;15:298-303. doi: 10.1007/s11926-012-0298-7. [ Links ]
![]() Correspondence:
Correspondence:
Juan Pérez Cajaraville
e-mail: jpcajaraville@hmhospitales.com
Recibido: 1-8-15.
Aceptado: 3-11-15.