DEFINITION OF OSTEOARTHROSIS
Osteoarthritis (OA) was classically defined as a degenerative joint condition characterized by progressive loss of articular cartilage, marginal bone hypertrophy (osteophytes), and changes in the synovial membrane, however today it is recognized that in this disease there is a protein and gene pattern of inflammatory characteristics similar to that found in diseases as diverse as rheumatoid arthritis or metabolic syndrome, for which reason the inflammatory component is currently recognized as a fundamental part 1. The Osteoarthritis Research Society International (OARSI) unifies concepts worldwide and defines OA as a disorder involving mobile joints characterized by cellular stress and degradation of the extracellular matrix initiated by micro and macro lesions that activate maladaptive repair responses, including proinflammatory pathways of innate immunity. The disease manifests itself first as a molecular disorder (abnormal metabolism of joint tissue) followed by anatomical and/or physiological disorders (characterized by cartilage degradation, bone remodeling, osteophyte formation, joint inflammation, and loss of normal joint function) that can culminate in illness 2. However, OARSI considers the need to develop a permanent process of standardized definitions of OA, thus helping to advance the development of drugs and research to promote understanding and treatment of this disease. That is why these definitions would be subject to regular refinement as new scientific advances demand it 3.
EPIDEMIOLOGY, PREVALENCE AND INCIDENCE OF HIP OA
Osteoarthritis (OA) remains a globally important public health problem, as described in OARSI's recent technical report, Osteoarthritis: Research Society. As noted in the document, OA affects 240 million people worldwide, about 10 % of men and 18 % of women over the age of 60 4. More than 80 % of those over 55 are Radiological OA, but only 10 to 20 % manifested some limitation of their activities due to OA. Among the symptomatic OA in peripheral joints, only 6 % have monoarticular symptoms, the rest are polyarticular. The frequency of involvement of the various joint groups are: knee 41 %, hands 30 %, hip 19 % 5. The prevalence of OA increases over time, being almost permanent in senescents aged 75 years or older. This condition is one of the main causes of physical disability in adults, affecting their financial situation and lifestyle. Increased life expectancy and an aging world population are projected to make OA the fourth leading cause of disability in 2020. Global data is sparse. In hip OA, values of 47 to 88 cases per 100,000 inhabitants have been reported, while incidences between 164 and 240/100,000 have been estimated for the knee 6.
The first estimates in the Spanish population, using clinical criteria for diagnosis, estimated the prevalence of OA in the urban population at 23.8 %, with a 2:1 female:male ratio. Authors point out that the appearance of hip OA is associated with personal factors, such as advanced age, ethnicity, comorbidities, density and considerable bone mass, and heredity. While there are joint factors such as polyarthrosis, carrying heavy loads, bone morphology and joint alignment. At the national level, according to data from the National Health Survey carried out in 2003, 3.8 % of the adult population reported suffering from osteoarthritis (self-report), the highest frequency among women and the highest in the measure that age increases 7.
PATHOPHYSIOLOGY
Osteoarthritis (OA) is characterized by the degeneration of the hyaline cartilage of the joints and is associated with the aging of the organism. One of the main characteristics is the slowness of its progression, which implies that the detection of loss of joint integrity is after years of evolution. The development of this pathology affects not only cartilage, but also the subchondral bone, joint capsule and peri-articular soft tissues, where in its final phase there is evidence of cartilage repair failure leading to degradation of the extracellular matrix (ECM), death of chondrocytes and total loss of cartilage integrity 8. The synovial membrane in the final stages of OA is said to develop an inflammatory response that contributes to the way pathology is expressed clinically, thus a patient with a severe OA condition closely resembles clinical expression from a patient with rheumatoid arthritis (RA), since similar changes are observed in the synovial membrane 9. OA has not been considered as an inflammatory arthropathy because they have not been found or there is a shortage of neutrophils in the synovial fluid, because it has the characteristic that the articular cartilage is avascular, it has no lymphatic drainage or innervation, so it is not possible to demonstrate the systemic manifestations of an inflammatory process as such, which prevents compliance with the classic signs of inflammation, however there are studies that demonstrate the existence of pro-inflammatory mediators such as cytosines (Interleukin - 1b and 6) and alfa Tumor Necrosis Factor (FNTalfa) which may be relevant in the development of OA 10.
The most common theory that attempts to explain the failure of cartilage is the loss of balance between anabolism and catabolism of the chondrocyte, which causes an imbalance between the synthesis and degradation of the extracellular matrix of articular cartilage. This leads to an accelerated destruction of the extracellular matrix (ECM) where the proteolytic enzymes of the chondrocytes themselves and synovial cells appear as those responsible, in addition there is an alteration in the cartilage repair systems 11. Proteoglycans are the majority component in the ECM, and are the first to be affected in OA, decreasing their concentration as the disease progresses. On the other hand, chondrocytes are not able to compensate for proteoglycan deficiency which results in a net reduction of the extracellular matrix. In earlier stages when the rupture of the surface layer occurs, small fragments of proteoglycans are released that come from the degradation of the same matrix to the synovial fluid, which stimulates the synthesis of IL-1b, IL-6 and TNF-a, among others pro-inflammatory mediators that act on cartilage inhibiting the synthesis of proteoglycans, further stimulating its degradation, which leads to a vicious feedback loop that perpetuates more and more inflammation of the membrane, causing irreversible fibrillation of articular cartilage 12. There are also hypotheses that suggest that the origin of OA is a consequence of a systemic disorder affecting the differentiation of stromal cells and lipid metabolism. This based on the fact that there is a close relationship between OA and obesity due to the common mesenchematic origin of the cells of the tissues that form the articular cavity, and the possible role of neuroendocrine mediators (such as leptin) in the regulation of mass 13. The chondrocyte plays a fundamental role in maintaining the integrity of the ECM of the articular cartilage, as well as in repairing the damage that the cartilage may suffer. OA is characterized by a change in the number of cells, which depends on the balance between cell birth (mitosis) and cell death. Cell death in its two forms, apoptosis and necrosis, is believed to be relevant in cartilage cell homeostasis. The difference between these two types of cell death is that apoptosis (cell suicide) does not trigger an inflammatory response since it is an active process that is under molecular control that also requires energy consumption. This energy is used to generate a disorder of the cellular structures, thus avoiding tissue damage, therefore, there is no inflammatory process 14. Studies show that nitric oxide (NO) plays a fundamental role in OA pathology, since its effect has been demonstrated in the inhibition of chondrocyte proliferation and in turn induces apoptosis in articular chondrocytes in humans. On the other hand, there are data that also show that the mitochondria suffer alterations in their respiratory chain (in an arthrosic chondrocyte) where they could also intervene in the apoptosis of the chondrocyte. Other studies mention that NO and some eicosanoids are involved in joint cartilage damage, specifically prostaglandin E2 (PGE-2) that affects remodeling, has a direct inflammatory action, may potentiate the effect of other proinflammatory mediators and the production of metalproteases. As well as leukotriene B4 (LT-B4) it also stimulates the release of cytosines such as IL-1b and TNF-alfa 15. Some studies mention that there is an increase in resorption at the beginning of the disease in patients with OA, which contributes to the loss of subchondral bone and also stimulates the production of some proteases such as cathepsin K and metalprotease. Bone to compensate for damage caused by bone resorption responds with the production of "new bone" and marginal osteophytes are the result of manifesting as nodules that can become inflamed or can irritate neighboring structures 16. Caspases are proteolytic enzymes that are activated in cellular apoptosis, these are divided according to their function; caspases that are involved with cytokine maturation (caspases 1, 4, and 5); effector caspases (caspases 3, 6 and 7) and initiator caspases (caspases 8, 9 and 10). The caspase cascade is believed to play an important role in the mechanism by which NO mediates apoptosis in chondrocytes. Together with NO, interleukin-1B (IL-1B) and tumor necrosis factor-alpha (TNF-alpha) induce modulation and expression of caspases in normal and arthritic chondrocytes 17. Other proteins involved in apoptosis are from the family of cell death proteins such as Bcl-2, which have implications for the survival of chondrocytes in OA. Finally, Fas ligation (LFas) is a cytokine responsible for regulating apoptosis since they are found in articular chondrocytes, which means that when this system is activated they contribute to apoptosis of the chondrocyte. It is in the superficial region of cartilage in OA where the greatest number of apoptotic cells are located and where Fas is expressed in chondrocytes, which bind to LFas, and induce chondrocyte apoptosis, however, at the level of the arthrosic synovial fluid, the concentration of LFas is very low. Chondrocytes have the ability to produce varieties of inflammation mediators, in turn the control of cartilage matrix deposition is not only related to the production of components of the ECM such as proteoglycans (PG) and type II collagen, As previously discussed, it is related to the balance between production and degradation. The function of the chondrocyte must be differentiated under normal and pathological conditions. First, under normal conditions, we know that its function is to maintain the balance between the substances it produces, but under pathological conditions, the chondrocyte responds to different stimuli, producing mediators of inflammation and enzymes that go to alter your normal metabolism. The proteolytic enzymes responsible for generating irreversible changes in normal joint architecture are Metalproteases (MMP). The activity that these enzymes develop is controlled by specific inhibitors (MMP tissue inhibitors) where 3 different forms are identified in the tissues of the human joint. TIMP-1, TIMP-2 and TIMP-3 which are present in cartilage and are synthesized under OA conditions by chondrocytes. There is an imbalance between the concentration of MMP and TIMP in the osteoarthritic cartilage and a relative difference of the TIMP is generated, this same condition has been found in the synovial fluid of OA 18.
Within the MMP families, stromelysins (MMP-3, MMP-10) and gelatinases (MMP-2, MMP-9) are those that generate greater severity in arthrosic cartilage and it has been shown that MMP-values 3 are extremely high compared to other MMPs in the synovial fluid of OA patients. Stromelysins have great capacity to degrade multiple components of the ECM, however, they can also activate inactive forms of other MMPs, therefore, they have a leading role in the destruction and progression of the cartilage matrix 19. There are 3 areas by which the articular cartilage is organized, these areas are the superficial, middle and deep and the cell density decreases from the superficial to the deep area. Half or one third of the cells in the superficial zone are represented in the deep zone, and adjacent to the deep zone is a calcified zone that is formed as a result of endochondral ossification. Of the 3 zones the one that is exposed to different tension, shear and compression forces is the superficial zone. This zone is made up of collagen fibers that provide it with greater strength to resist the different forces it is subjected to, and also flattened cells that are located parallel to each other in the superficial layer of cartilage. It is worth mentioning in a very important way that the calcified area is isolated from the most superficial layers, so the low repair capacity is a product of the low irrigation of the most superficial tissue. There are two conditions by which the OA is developed; first when the biostructural properties of cartilage and subchondral bone are normal, but there is an excess of articular load which leads to tissue changes; second when the load is normal but the biostructural structures are deficient 20.
CLINICAL PRESENTATION OF HIP OA
OA is very likely to have a long asymptomatic period and is therefore difficult to detect in the early stages. Patients come for consultation when pain appears with progressive functional limitation, constituting a common reason for medical consultation and a frequent cause of deterioration in lifestyle. There are studies that show that up to 50% of people with symptomatic OA suffer some degree of disability 21.
The main clinical manifestations associated with OA are: joint pain, stiffness after inactivity, mainly pain. Joint pain in OA is relieved by rest but increases with resumption of activity. Osteoarthritic knee pain is typical, which is exaggerated when starting to walk after rest and is relieved after walking a little. Locations lead to different clinical pictures 9. In the hands, in addition to pain, Heberden and Bouchard nodules may appear, in the distal and proximal interphalangeal joints, respectively, or rhizarthrosis when it is in the metacarpophalangeal joint of the thumbs. Knee OA is a typical case of disability. In addition to pain and inflammation, crepitus and muscle atrophy may appear. Hip OA is characterized by pain localized to the hip itself or radiating to the thigh and knee. In the spine it can appear in the cervical, dorsal and lumbosacral segments, affecting the intervertebral discs, the vertebral bodies and the apophyseal joints 22.
The diagnosis of OA is essentially clinical. Radiological findings can also be very useful in evaluating anatomical changes, possible complications, and the degree of disease progression, but they do not always correlate with the degree of symptomatology or joint dysfunction. In general, the radiological signs in all osteoarthritis are: narrowing of the joint space, presence of subchondral sclerosis, subluxation, cysts and osteophytes 23.
Bone scintigraphy (Nuclear Magnetic Resonance) is recommended when considering an osteotomy and requiring knowledge of the actual situation of the joint's external behavior. Arthroscopy is a technique that allows a more detailed description of the depth and extent of the lesion, as well as the very early detection of softening and fibrillation 24.
TREATMENT OF HIP OA
Currently, there is no treatment that reverses and heals OA, only therapies are available whose therapeutic objective is to control and improve symptoms such as pain, functionality and delayed disease progression. In this sense, considering the available clinical evidence, the European League Against Rheumatism (LECR) and the International Society for Osteoarthritis Research (SIIOA), developed a series of recommendations to combat OA, mainly based on using pharmacological and non-pharmacological measures, being individualized with the characteristics of each patient, the joint involved, the clinical involvement and the existence of other underlying pathologies 25.
PHARMACOTHERAPY
Among the drugs most commonly used to counteract the symptoms of OA are non-steroidal anti-inflammatory drugs (NSAIDs) and cyclooxygenase enzyme inhibitors (COX), which, although they do not prevent cartilage damage, help to relieve pain and inflammation. Nonspecific NSAIDs (aspirin, diclofenac, ibuprofen, indomethacin, piroxicam, among others), non-specifically inhibit COX-1 and 2 with greater effect on the former, however they produce various adverse events (AE), among which stand out gastrointestinal symptoms induced by COX-1 inhibition leading to reduced synthesis of prostaglandins (gastroprotective factors of the gastric mucosa), increased blood flow to the gastric mucosa, and increased acid secretions and oxidative stress (OS). The introduction of COX-2 inhibitors was intended to produce similar effects with a reduction in gastrointestinal AEs, however, there is evidence that these agents increase cardiovascular risk, a frequent condition in older patients that is most sensitive to OA and receiving treatment with NSAIDs, therefore, its use has been limited, even being contraindicated by the European Medicines Agency in patients with ischemic heart disease or stroke, so they should be used with caution in cases of hypertension, hyperlipidemia, diabetes and in smokers 26.
For their part, COX-3 inhibitors, such as acetaminophen, do not present these risks, making it the oral analgesic of first choice, however its anti-inflammatory effect is low, and higher doses can be associated (> 10 g/d) at the risk of liver complications and increase the effect of anticoagulants.
Regarding the topical use of NSAIDs, it allows reducing AE and minimizing systemic toxicity, but according to a meta-analysis it is not effective in the long-term treatment of this pathology 27.
Considering all the above aspects, paracetamol is the first pharmacological option suggested in the OA management guides of the European League against Rheumatism, as well as the American College of Rheumatology, while the International Society for Research in Osteoarthritis (ISROA) recommends the use with caution in the case of hypertension, hyperlipidemia, diabetes and in smokers for both COX-2 inhibitors and the rest of NSAIDs. It is worth mentioning that the prescription of NSAIDs must be carried out in a personalized way for each patient, taking into account the characteristics, preferences and cardiovascular and gastrointestinal risk factors of each individual, as well as the overall safety profiles of each medication 28.
On the other hand, opioid treatment is recommended in patients who are not candidates for surgery, as an alternative to NSAIDs, when they are unable to control pain, or have contraindications or intolerance 25,26,27,28,29. In Chile, 2 opioid analgesics are used that can be used orally: codeine and tramadol, indicated as monotherapy or in combination with paracetamol, the risk-benefit evaluation being important in these cases, considering the potential risk of dependency. On the other hand, transdermal fentanyl and buprenorphine have a good safety / efficacy profile if they are correctly prescribed for the treatment of chronic pain 30.
NON-PHARMACOLOGICAL TREATMENT
This type of treatment includes the education of the patient and his family, an exercise program, among others. The delivery of information and education is an essential component of the management of physical therapy, being an obligation of medical teams, as well as a shared responsibility with patients. This is particularly important in chronic diseases such as OA and should include aspects related to the importance of the disease, its forms of study, therapeutic alternatives, and prognosis. In this sense, individual or group educational programs, periodic telephone calls and training in techniques to cope with the problem are useful, with an effect size that ranges between 0.28 and 0.35 31.
In the case of hip OA, an RCT of patients expecting hip joint replacement showed that patients receiving group education had less pain than the control group. The fundamental objective of health education is to promote a better understanding of OA and to promote self-management strategies for this degenerative disease 32. Inclusion in weight reduction programs can help, with dietary recommendations and stretching and aerobics (walking, cycling) to increase strength and muscular endurance, aiding weight loss 33.
Current clinical guidelines recommend exercise therapy in the treatment of hip OA, incorporating exercises such as hip stretching, strengthening exercises, and balance tasks. Although OA is a progressive and degenerative disease that can naturally worsen over time regardless of intervention, the best available evidence seems to favor exercise therapy for short-term pain relief, requiring more RCTs to confirm the duration of the effect. In addition, these exercise programs must be personalized and should be introduced gradually as the patient is able to tolerate it 34.
On the other hand, the use of canes that promote the reduction of joint overload, or footwear with a rubber sole and heel or other external devices, that modify the functional or structural aspects of the musculoskeletal system (insoles, external wedges, knee pads, etc.) may decrease the forces applied to the joint 35.
As for the cold, ice massages can relieve pain in the OA, improving flexion and functionality; while the application of heat relaxes the muscles, decreases the sensation of pain and improves morning joint stiffness. The use of transcutaneous electrical stimulation can control pain in patients with osteoarthritis of the hip who are not candidates for pharmacological treatment, with a minimum application of 4 weeks being recommended. Other therapies such as electrical muscle stimulation, ultrasound, short wave, laser and magnet therapy have been tested, without sufficient evidence on their efficacy 36.
MANUAL THERAPY IN THE HIP OA
Manual therapy is a physical treatment used by physical therapists, chiropractors, osteopaths, and other practitioners to treat musculoskeletal pain and disability, which includes massage therapy, joint mobilization, and manipulation. Recently published clinical guidelines on the treatment of OA recommend manual therapy as an adjunct treatment (NICE, 2008; RACGP, 2009), however this recommendation was based on a single study.
And it is that in several studies and reviews it can be observed that there is no clear description of what constitutes manual therapy, evidenced in the different criteria they use for the inclusion of studies. That is why some authors use a broad definition of manual therapy.
Regarding the effects of the techniques on pain, Skyba et al. showed that they have a role in activating the cortical inhibitory pain system in addition to the release of endorphin, the increase in blood flow that can release local pain mediators. and also psychological influences through the interaction between the doctor and the patient 37.
METHODS
ELIGIBILITY CRITERIA
The criteria established by the authors of this panoramic review were; systematic reviews published between 2000 and 2017, reviews in spanish or english language, reviews comparing manual therapy intervention as treatment of hip osteoarthritis compared to some other non-surgical treatment modality, which reviews have included RCTs with an established and declared randomization, that the reviews have included patients older than 18 years and with an imaging diagnosis of hip OA.
ELECTRONIC SEARCH
This panoramic review or Overview considers systematic reviews with or without meta-analyzes, written in Spanish or English, published between the years 2000 and 2019. A systematic search was carried out in electronic databases, in order to compile the available literature on the subject to be treated, taking as reference the PRISMA statement for systematic reviews 38. The search process was carried out in the following databases; MEDLINE, SCIELO, SPORT DISCUS, CINHAL, SCOPUS and GOOGLE SCHOLAR, until January 15, 2020, using as search terms "Multicomponent therapies", "Manual therapy", "hyposteoartrithis", "hip joint" and "therapies osteoarthritis", for which the following Boolean connectors" AND "" OR "and" NOT "were used, of which the search algorithm is shown in Figure 1.
INCLUSION CRITERIA FOR THE SEARCH
They included RCTs and systematic reviews that in their abstract or title presented any of the search terms mentioned above, it is also considered or that the article was available for full-text analysis, no sample size restriction was applied in any of the articles. Exclusion criteria were letters to the editor, bibliographic reviews, and articles published in the gray literature.
RISK OF BIAS FOR PRIMARY STUDIES IN REVIEWS
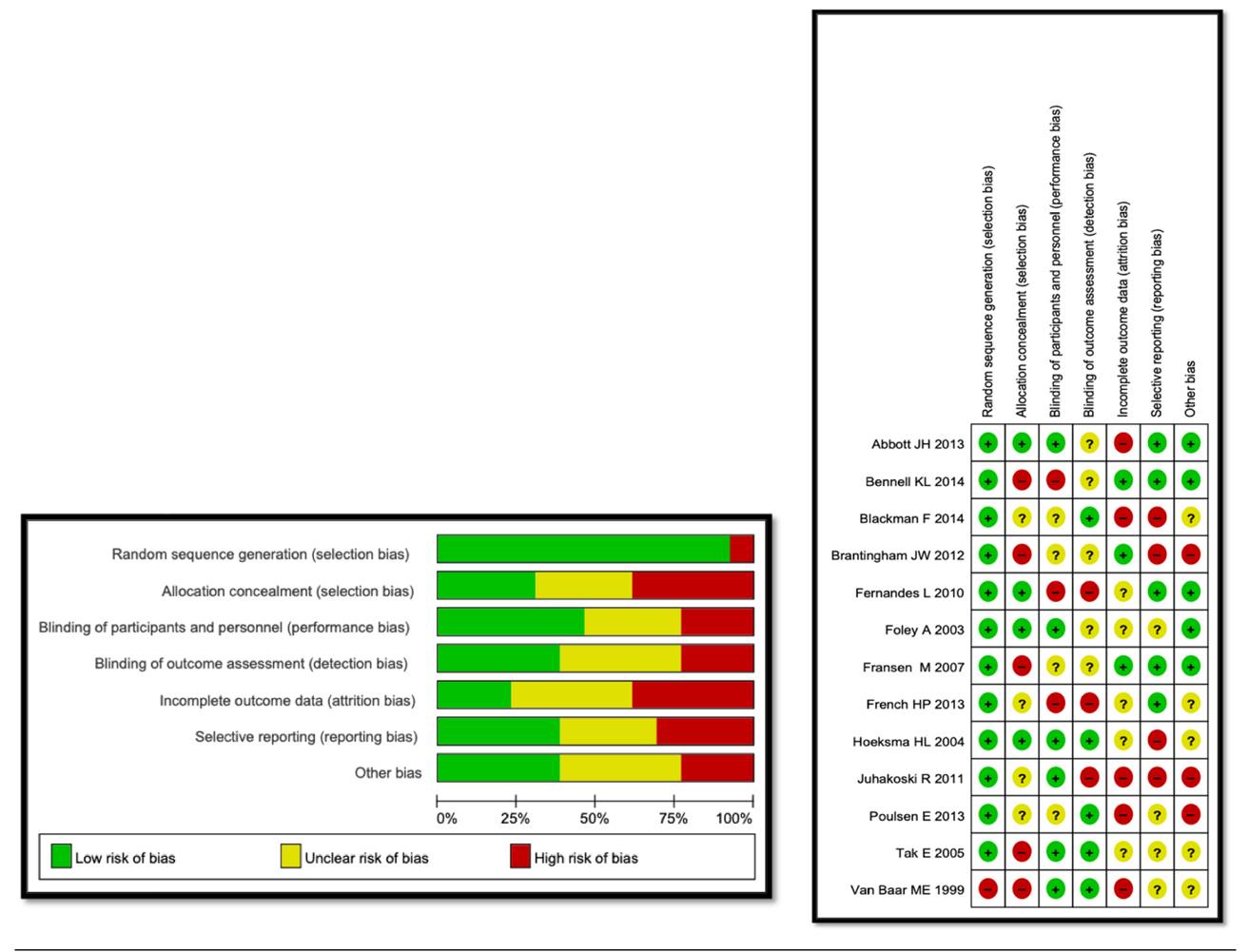
The risk of bias assessment of the primary studies included in the reviews included in this panoramic review was performed (Figure 1), for the individual bias analysis it was performed as recommended by the Cochrane Collaboration Manual 39. This tool assesses the risk of bias according to seven domains: random sequence generation, randomization sequence concealment, blinding of participants and treatments, blinding of outcome evaluation, incomplete outcome data, selective outcome reporting, and other biases. Each domain could be considered as low risk of bias, unclear risk of bias or high risk of bias.
RESULTS
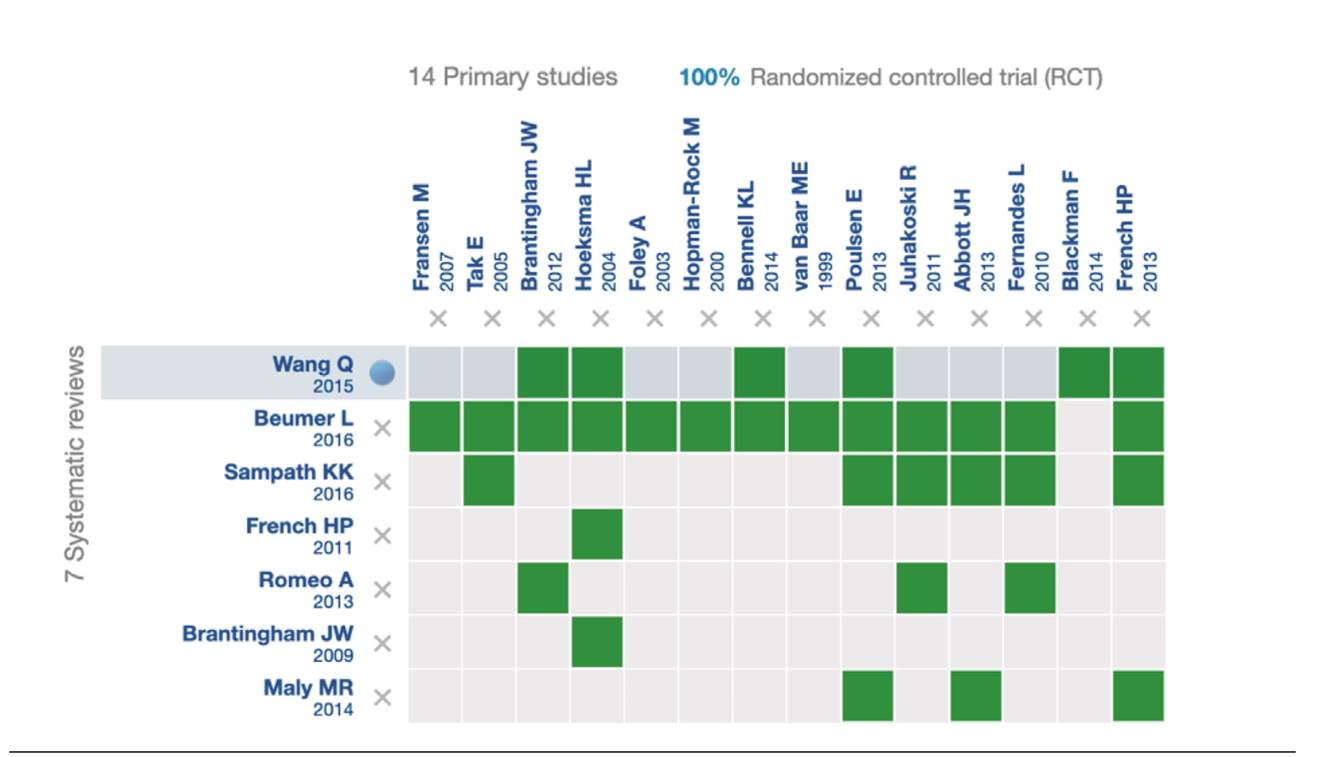
After reviewing 30 titles and abstracts, we included 7 systematic reviews and 14 primary studies (Flow diagram). Figure 2 provides details of the clinical trials used in the systematic reviews included in this panoramic review. Table 1 summarizes a series of data extracted from the systematic reviews included in this panoramic review, such as the number of included studies, number of citations, and if these are cited by any review included in this panoramic review, whether or not they report at least one meta-analysis, and report risk of bias. Table 2 summarizes all the outcomes used by the included systematic reviews.
Table I. Description of the clinical trials used in the systematic reviews included in this overview

RISK OF BIAS PRIMARY ARTICLES IN THE REVIEW
When using the Cochrane Collaboration tool to assess the risk of bias of all clinical trials, it was found that 7.69 % had a low risk of bias 36, 46.14 % had moderate risk of bias 40-43 and 46 % 14 had a high risk of bias (36,38) (Figure 3).
EFFECT OF MANUAL THERAPY ON PAIN INTENSITY
Seven randomized clinical trials were identified that have measured the pain intensity of hip OA by subjecting subjects to a MT intervention compared to a control group of which 4 34,36,37,40,41,42,43,44,45,46,47 used the Visual Analog Scale, where they had to indicate their pain intensity in a line from 0 to 100 mm, 2 used the WOMAC index for part of the pain 36,45. The WOMAC index measures five elements for pain (score range 0-20), two for stiffness (score range 0-8) and 17 for functional limitation (score range 0-68), where in the pain section are rated on a scale of 0-4, which corresponds to: None (0), Mild (1), Moderate 2, Severe 3) and Extreme, with a possible score range of 0-20 for the pain; and only the study by Paulsen et al. used the Numerical Rating Scale (NPRS) from 0 to 10 (where 0 indicates no pain and 10 indicates the worst possible pain).
In the study by Paulsen et al 37, pain intensity was measured with the NRPS, in a control group (36 individuals) and in a MT group (34 individuals), and as results they found that the intervention group had, on average, a score of 4 (SD: 2.5) in the NPRS in contrast to the average of 4.9 (SD: 2.5) in the control group. The studies by Hoeskma et al., French et al., Bennell et al., and Blackman et al., they used the Visual Analog Scale, of which they found a greater decrease in the control groups when compared with the MT groups (Hoeskma et al. MT group: 36.7 (SD: 44), control group: 32.4 (SD: 35); French et al. MT group: 4.2 (SD: 3.42), control group: 4.02 (SD: 2.88); Bennell et al. MT group: 43.7 (SD: 24.8), control group: 39.4 (SD: 25); and Blackman et al. al. MT group: 43.8 (SD: 24.6)) (Table 3). Finally, only two studies 36 used the WOMAC index of part of the pain, to assess the intensity of the pain where their results show a superior effect of the MT compared to the control group. The study by Brantingham et al., the MT group obtained 122 (SD: 73) compared to the control group that obtained 136 (SD: 100) in the pain intensity evaluated by the pain part of the WOMAC index. On the other hand, the study by Abbot et al. the MT group obtained 13.7 (SD: 11.89) compared to the control group that obtained 15.52 (SD: 11.75) (Table 3).
EFFECT OF MANUAL THERAPY IN CONJUNCTION WITH EXERCISES ON PAIN INTENSITY
Four randomized clinical trials were identified that have measured the pain intensity of hip OA when subjecting subjects to an intervention with MT + Ex in conjunction with exercises, within which 2 studies used the Visual Analog Scale (34,36,37,40,45,46) to measure the intensity of pain. The remaining 2 studies used the NPRS 37 and the WOMAC pain section (36) (Table 4). The study by French et al. obtained as results using the VAS in the MT + group Ex 42 (SD: 34.2) compared to 56.2 (SD: 28.4) in the control group; the second study using the VAS was that of Bennell et al. who obtained as results in the MT + group Ex 46.8 (SD: 26.7) and in the control group 43.1 (SD: 27.3). On the other hand, the study by Paulsen et al. used the NRPS to assess the intensity of pain and obtained in the MT + group Ex 18 (SD: 31) compared to the control group which obtained 10 (SD: 20). And finally, the study by Abbot et al. they occupied the WOMAC index, which was obtained in the MT + group Ex 18.57 (SD: 12.56) compared to 15.52 (SD: 11.75).
EFFECT OF MANUAL THERAPY ON FUNCTIONALITY
Seven randomized clinical trials were identified that measured Functionality in subjects with hip OA when subjected to a MT intervention comparing them with a control group. The study by Paulsen et al. 37 used the Hip Disability and Osteoarthritis Scale (HOOS) consists of 40 items, selected from 51 original items that evaluate five separate dimensions relevant to the patient: pain (ten items); Symptoms including stiffness and range of motion (five items); Activity limitations: daily life (17 articles); Sports and recreation function (four articles); and hip-related quality of life (four items) in which a score of 80 (SD: 17) was obtained in the MT group in contrast to 69 (SD: 18) (the less, the more functionality), see Table V. The study by Hoeskma et al. he measured functionality with the Harris Hip Scoring Scale (HHS), which contains ten elements that cover four domains, which are pain, function, absence of deformity and range of motion. HHS is divided into three sections. The first section are questions about pain and its impact that are answered by the patient or client. The second and third sections require the physical therapist to evaluate the hip joint and function of the patient or client. HHS is a measure of dysfunction, so the higher the score, the better the result will be for the individual. The maximum possible score is 100. The results can be interpreted with the following: <70 = poor result; 70-80 = fair, 80-90 = good, and 90-100 = excellent 47. In their study, Hoeskma et al found a score of 70.2 (SD: 20) in the MT group, and 59.7 (SD: 18) in the control group (Table 5). On the other hand, the study by Blackman et al. used the Lower Limb Functional Scale (LEFS) to measure functionality in subjects with hip OA, which is a self-report questionnaire. Patients respond by choosing one of the 5 options for the different scenarios of the question "Today, do you have or would you have any difficulties with: 0 points = Extreme difficulty or unable to carry out activity, 1 = Fairly difficult, 2 = Moderate difficulty, 3 = A little of difficulty, 4 = No difficulty ", and whose maximum possible score is 80 points, which indicates a very high function, and its minimum possible score is 0 points, which indicates a very low function in which, they had as results 4 (SD: 9.32) in the MT group and 6.36 (SD: 14.29) in the control group (Table 5) (47). The other 4 included studies used the function section of the WOMAC index, the study by French et al. were obtained as a result in the MT group 29.31 (SD: 17.06), and in the control group 28.08 (SD: 15.48). In another study by Bennell et al. they obtained 27.5 (SD: 12.9) points in the MT group, and 26.4 (SD: 11.3) in the control group. In the study by Abbott et al. 52.25 (SD: 41.12) points were obtained in the MT group, and in the control group they found 63.74 (SD: 45.22). Finally, the study by Brantingham et al. 740 (SD: 561) points were obtained in the MT group, and 619 (SD: 405) points in the WOMAC index 46) (Table 5).
EFFECT OF MANUAL THERAPY IN CONJUNCTION WITH EXERCISES ON FUNCTIONALITY
Regarding the effects of MT + Ex on functionality, we found 2 randomized clinical trials included in the reviews reviewed in this study (34,36). The study by French et al. used the functionality section of the WOMAC index, in which they obtained 29.31 (SD: 17.06) points in the MT + Ex group, and in the control group 36.09 (SD: 16.41) points. And in the study by Abbott et al. using the same functionality section of the WOMAC index, in which it obtained in the MT + group Ex 67.9 (SD: 46.13) points, compared to 63.73 (SD: 45.21) points in the control group (Table 6).
ANALYSIS OF RESULTS THROUGH FOREST PLOT
The results included in the reviews of this study in order to qualify for meta-analysis of each of the systematic reviews, were grouped into different outcomes for the analysis through the forest plot, which shows us whether they are statistically significant for the intervention group or towards the control group, the first analysis focused on quality of life for patients with OA, the results reported by this statistical analysis favored the intervention group with manual therapy overall, but these were not statistically significant towards said group (Figure 4). The second analysis was carried out to measure the decrease in pain with MT, the results presented by the forest plot analysis for this outcome, show that the overall changes favored the control group, but the results were not statistically significant in favor of the group of intervention with MT (Figure 5). The third analysis measured the functionality, unlike the previously exposed outcomes, the functionality outcome did not present an overall change towards either of the two groups, showing no statistically significant differences for any of the previously exposed groups (Figure 6). For the overall perception of MT by the patients included in the studies, they did not have a clear preference neither in favor of the experimental group nor towards the control group (Figure 7). Finally, the analysis of adverse effects with the interventions had an overall effect towards the control group, but this change or inclination was not statistically significant.
CLINICAL AND STATISTICAL HETEROGENEITY
Our review describes different parameters and results in the data evaluation, which generated marked statistical heterogeneity, which is attributed to the fact that not all systematic reviews presented significantly statistical data in their results, the above was presented for the intervention group and for the control group. This did not allow any of the systematic reviews to be meta-analyzed, leaving enough questions to determine the correct effect of the manual therapy intervention in hip OA. From the point of view of clinical heterogeneity, this was to a lesser extent since the majority of the RCTs included in the systematic reviews analyzed similar clinical outcomes, the interventions were quite homogeneous, the patients did not show marked differences, and the groups of intervention as control groups were quite homogeneous, this can be attributed to the fact that the reviews included in this study presented similar primary studies among them.
DISCUSSION
This panoramic literature review provides an overview of the systematic reviews included in the literature on the scientific evidence for the conservative management of hip osteoarthritis, the studies analyzed 32,33,34,35,46,48,49 reported in favor of manual therapy, but due to the lack of statistical power of the results, these cannot be categorical as the standard conservative intervention in the management of hip OA. Osteoarthritis of the hip is a complex condition that often requires surgical management, which is always a complex conditioner for patients and for the management of the deleterious condition to any type of surgery 43,50. Therapeutic exercise in the most common hip OA of physical therapy and manual therapy 49. For this intervention, the results of our panoramic review evaluated a varied number of outcomes that are part of a global therapeutic evaluation or the Gold standard for this type of patients, although what was expected was to be able to find some absolute guidelines for therapeutic management, the statistical heterogeneity presented suggests that manual therapy is a useful complement, but not absolutism, for the conservative management of this type of patient. If we analyze in detail because the studies show some differences, it is because a high number of the RCTs included in the systemic reviews had a high and medium level of bias, which could also mean that these data cannot be so reliable, therefore, they cannot be interpreted in the same way. The only RTCs included in the systematic reviews was that of Abbott JH, et al, which presented only one level of the scale of bias poor, when comparing the results with the other RCTs, we can interpret that their results based on the outcomes described, if they were in favor of manual therapy, but compared to the control group, these were not statistically significant like the other RCTs 36. To our knowledge, this is the first panoramic review comparing the effect of manual therapy on hip osteoarthritis, therefore we propose to carry out new primary studies to eliminate some biases in program execution and improve intervention in the control group, such as in the intervention group.
Finally, it is necessary to mention the limitations of our study are as follows: although we searched six databases and included articles in 2 languages, we could have lost relevant articles for our search; there was a high degree of statistical heterogeneity among the studies including the possible sources of heterogeneity could be variations in the type and dose of the interventions used, and the results measured; methodological limitations such as the lack of an adequate sample size, the unclear randomization, improperly concealed allocation, and lack of blinding of assessors could overestimate the effect size of the interventions studied; due to the limited number of included studies, publication bias could not be assessed.