Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de la Sociedad Española del Dolor
versão impressa ISSN 1134-8046
Rev. Soc. Esp. Dolor vol.28 no.1 Madrid Jan./Fev. 2021 Epub 29-Mar-2021
https://dx.doi.org/10.20986/resed.2021.3841/2020
Review
Inflammatory mediators: its connection with chronic pain and associated problems. Review
1Facultad de Fisioterapia. Universidad de Vigo, Pontevedra, Spain
2Facultad de Ciencias de la Educación y del Deporte. Departamento de Didácticas Especiales. Universidad de Vigo, Pontevedra. Spain
3Grupo de investigación en Educación, Actividad Física y Salud. (Gies10-DE3). Instituto de Investigación Sanitaria Galicia Sur (IIS Galicia Sur), SERGAS-UVIGO. Vigo, Spain
INTRODUCTION
The cause of musculoskeletal pain has historically been associated with an injured tissue that sends painful neuronal inputs to the central nervous system for pain perception. However, there should not always be a relationship between pain and nociception, or between pain and tissue damage 1. Currently, the IASP (International Association for the Study of Pain) says that "pain is an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage" 2.
Pain is defined as a complex interaction of homeostatic systems in response to an identified threat 3. Therefore, pain should be considered as a process that integrates sensory, cognitive and/or emotional information from actual or potential threats 4. This threat assessment process is performed by a set of cortical and subcortical structures called pain neuromatrix 5. However, we must differentiate acute pain from chronic pain (CP). The basis of acute pain is the inflammatory response, that is, a harmful stimulus or tissue damage leading to the release of chemical algogens, such as prostaglandins, bradykinin, tumor necrosis factor (TNF), neural growth factor, histamine, substance P and the calcitonin gene-related peptide. This inflammation will sensitize the nociceptor and increase the generation and transmission of stimuli, known as peripheral sensitization, decreasing the nociceptive threshold and facilitating nociceptive responses to promote tissue recovery 6. In contrast, CP is characterized by sensitization of the spinal cord, activation of the nociceptive pathways projecting into the spinal cord and midbrain, and activation of the descending pain facilitation systems and loss of descending inhibition, for the maintenance of sensitization 7.
People with emotional problems, behavioral problems, excessive alcohol use, or sleep disorders have been shown to be more likely to develop musculoskeletal pain in the medium or long term 8. In fact, CP has a devastating effect on many aspects of daily life, reducing the patient's quality of life by negatively impacting on the physical and emotional health 9. According to a European survey, half of patients with CP feel tired all the time and 40% patients feel helpless or unable to think or function normally 10. In addition, 62% patients reported a lack of awareness and knowledge of the disease in their environment, and 47% patients believed that the rest of the people doubted the real existence of their pain 9.
A clinical entity, generalized chronic pain (GCP), is defined as generalized body pain, and often includes other somatic and psychological symptoms such as depression and anxiety, lack of sleep, fatigue, and decreased cognitive ability 11. Therefore, beliefs and emotions are capable of activating neuromatrix, provoking and even perpetuating pain without the need for nociception 12.
Sleep problems, including insomnia, are a common complaint among adults with CP 13. These alterations have been associated with higher levels of circulating inflammatory cytokines 14, which also vary in response to stress 15. In addition, various research studies have confirmed the positive correlation between catastrophism, pain intensity and perceived disability 16,17.
Similarly, increased sensitivity to experimental pain (hyperalgesia) has been found in conditions of CP. Compared with healthy subjects, patients with CP have low pain thresholds, which implies a change in perceived sensations with increased pain after a non-painful stimulus (allodynia), decreased pain tolerance and increased pain rates in response to experimental pain stimuli 18. In this sense, generalized hyperalgesia has been associated with C-reactive protein (CRP), revealing an association between CRP and experimental pain tolerance 19. In addition, after peripheral nerve injury, macrophages and Schwann cells, which gather around the injured nerve area, release cytokines and specific growth factors needed for nerve regeneration. Localized inflammatory irritation of the dorsal root ganglion (DRG) not only increases proinflammatory cytokines, but also decreases anti-inflammatory cytokines 20,21.
CRP can be synthesized in the liver and other cells of tissue such as the kidney 22, lung 23, nervous system 24, adipocytes 25 and leukocytes 26. CRP is expressed as a result of several stimuli, such as IL-6 and IL-1, being considered a classic acute phase protein . The level of CRP is acceptably correlated with the severity of inflammation, making it a reliable marker of infections, inflammation, and response to treatment 27 and therefore an inflammatory marker associated with pain.
In summary, although acute pain is traditionally related to inflammation, the aim of this study is to analyze the behavior of inflammatory mediators (IM) in diseases involving chronic pain and disorders associated with that disease, so that we can understand the long-term pain and all the mechanisms influencing it, so that we can approach it more appropriately.
MATERIAL AND METHODS
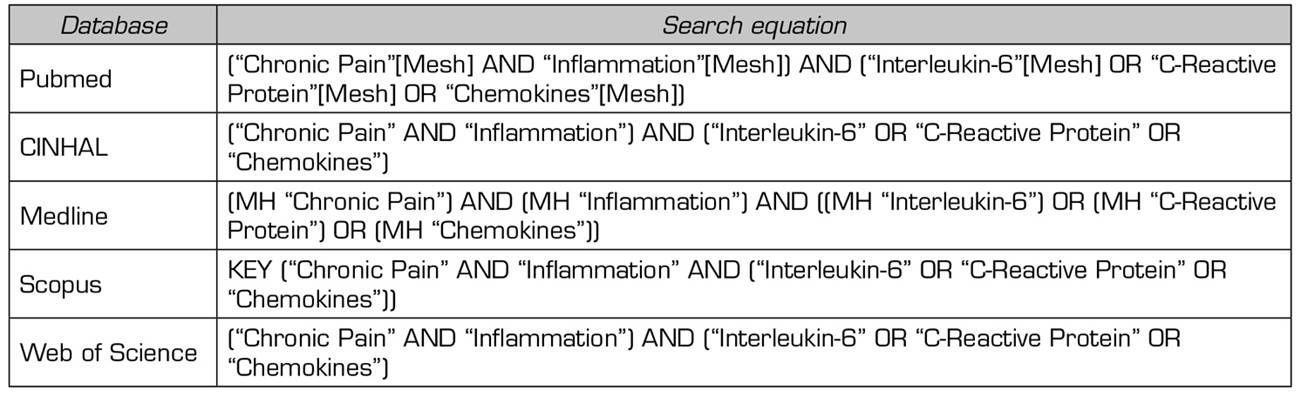
A search was performed on PUBMED, Scopus, Web of Science, Medline and CINHAL in January 2020 to conduct the present study. The same Medical Subject Headings (MeSH) terms were used in the used databases: "Chronic Pain", "Inflammation", "Interleukin-6", "C-Reactive Protein" and "Chemokines" (Table 1).
To obtain valid results, the inclusion criteria were studies published in the last 5 years and written in English or Spanish. The exclusion criteria were: Review studies, articles repeated on other databases or dealing with another topic. The results and the search process are summarized in Figure 1.
RESULTS
In the presentation of the results, a table has been created with the aim of facilitating the understanding of methodological characteristics. In addition, it has been sought to establish a logical order based on the different analyzes and variables to facilitate their understanding and subsequent analysis. These methodological characteristics are described in Table 2.
Among the main results of the articles included, Sibille et al. 28 noted that CRP was significantly higher among individuals with CP compared with those without CP (p < 0.001).
Likewise, Feinberg et al. (29) noted that the mean CRP was significantly higher in cases of CP (Ẋ = 5.54 mg/l) compared with controls (Ẋ = 3.75 mg/l). Serum level of CRP showed a positive association with CP (p = 0.0001).
Bäckryd et al. 30 started from studies that related central sensitization in neuropathic pain to the presence of increased values of proinflammatory cytokines. They found higher IL-6 values compared to the control group. In contrast, no significant differences in IL-1β and IL-8 were found.
In patients with lower back pain, Teodorczyk-Injeyan et al. 31 found that baseline IL-1β, IL-6, and TNF levels were all significantly higher in both groups of patients with lower back pain compared with asymptomatic controls (p = 0.0001 to 0.003). TNF production was significantly higher in patients with chronic low back pain versus control and acute low back pain (p = 0.003). In patients with chronic low back pain, IL-1β and TNF levels correlated positively with assessed pain scores (p < 0.001). In both patient groups only IL-6 levels were significantly correlated with ODI Disability Scale scores (p<0.001).
Hysing et al. 32 tested 92 proteins. CRP values were obtained from 45 patients and had an average value of 2.6 mg/l, which they classified as normal. Baseline values of a total of 39 inflammatory markers of two cysteine (CC) and chemokine CXC types, belonging to the IL-8 group, were higher in the 28 patients who participated in the intervention during one year of behavioral therapy. Furthermore, they found lower values before treatment in the following 4 cytokines, TNF, cluster of differentiation 6 (CD6), IL-18 and oncostatin M (OSM).
Lasselin et al. 33 also applied behavioral therapy completing 12 group sessions with a duration of 90 minutes each, obtaining a significant decrease in serum TNF concentrations (p = 0.02), but not in IL-6 or IL-8. Instead, treatment was found to reduce psychological unhappiness (PIPS) and improve mental health-related quality of life (SF-36), but only in subjects with low baseline inflammatory status compared to other participants.
Bäckryd et al. 34 aimed to relate the risk of peripheral neuropathy and the association of increased cytokine levels. They analyzed 50 proteins, of which 10 % were significantly associated with peripheral neuropathy compared with the control group. These include IL-8, GC, growth factor-related cytokines, and inflammatory factors.
Regarding the study conducted by Gerdle et al. 11, pain thresholds in the upper and lower limbs were measured with different pressure, cold and heat stimuli, to record the intensity of the pain and analyze its influence on inflammatory proteins. They have found significant differences in pain intensities between groups. In addition, both groups showed a poor quality of life, with a mean of about 38 points on the scale used. Regarding the cytokines analyzed, 11 proteins had significant differences between the groups, including IL-1β (p = 0.026), different types of chemokines such as CCL20 (p = 0.042), CCL28 (p = 0.051) and CCL4 (p = 0.062), of which only the CCL20 obtained significant values.
Chamessian et al. (35) showed significantly higher levels of TNF, IL-8, CRP and growth factor-related proteins. In addition, mean pain severity scores (S-LANSS) and the pain catastrophizing scale (PCS) significantly correlated (p < 0.01) with TNF, IL-8, and growth factor-related proteins.
Schistad et al. (36 aimed at analyzing the variability of the high-sensitivity C-reactive protein (hs-CRP) in patients with chronic pain, and also how cold tolerance influenced it. They analyzed anxiety and pain with the Hopkins Symptom Scale (HSCL), but found no significant differences between groups. Participants with chronic pain achieved a significantly higher level of hs-CRP (with hs-CRP > 3 mg/L in 18.5 % of participants) compared with those without pain (hs-CRP < 3 mg/L in 1.7 %). The authors observed a negative relationship between hs-CRP levels and cold tolerance, as 70.1% of people who were in the mild hs-CRP level (< 1,0 mg/l), 69.1% of patients with an intermedium level (1.00-2.99 mg/L), 63.9% of those with a slightly high level (3.00-10 mg/L), and only 61.2% of those with high levels of hs-CRP (> 10 mg/L) completed the total test time.
Skarpsno et al. 37 analyzed the risk of chronic pain based on hs-CRP and insomnia levels. 18% of people with hs-CRP< 1 mg/L are associated with high insomnia, and 12% have chronic pain. At levels of 1.00-2.99 mg/L hs-CRP, 21 % reported sleep disorders and 27 % had chronic pain. In hs-CRP>3.00 mg/l they had symptoms of which 31 % was related to lack of sleep and 23 % to chronic pain.
Heffner et al. 38 based their objective on improving insomnia with 6 weeks of cognitive behavioral therapy (CBT-I) and evaluating their subsequent influence on IM in patients with osteoarthritis of the knee. They showed that men (Ẋ = 16.33 points) reported a higher severity of insomnia than women (Ẋ = 24.27 points), but this difference was not statistically significant (p = 0.04). At 8 weeks of follow-up, insomnia severity scores in the CBT-I group were significantly lower than in the control group (p = 0.001). They compared insomnia improvement with the Western Ontario and McMaster Universities Osteoarthritis index (WOMAC), Knee Pain Scale (KPS), Cold Pressure Test (CPT), and IL-6 and TNF through the initial pain assessment session. WOMAC scores decreased in the experimental group by 9.06 points, suggesting a significant change in the signs and symptoms by improving insomnia. The response of IL-6 in relation to insomnia improvement time was significant (p = 0.001). Participants who had improved insomnia during follow-up showed significantly reduced IL-6 levels.
Park et al. 39 analyzed IM in patients with temporomandibular disorders (TMD) and how insomnia influences these values. RCP values were higher in the group with more pain, however, differences between groups were not statistically significant (p > 0.05). However, the levels of IL-1β, IL-6 and TNF were significantly different between the groups (p < 0.001 for all). The group more pain had the highest levels of all the proinflammatory cytokines analyzed. Significant differences were found between the more pain group and the control group for IL-6 (p < 0.01) and between the lower pain groups and the control group for TNF (p < 0.01).
Sleep scores on the sleepiness scale differed significantly between groups (p < 0.001), with the highest scores in the more pain group.
DISCUSSION
All the articles included in the present review are observational studies, because this is a topic that is in the early stages of research.
Thanks to the tools used, high IM levels have been significantly correlated with high levels of disability 31, psychological unhappiness 33, poor quality of life 33, high pain intensities and severity 11,35, high catastrophizing dates 11,35 and insomnia 37,38,39. Therefore, the association of chronic pain with comorbidities is present and increases the risk of suffering it.
PAIN AND IM
There is evidence showing that IL-6 contributes to the development of neuropathic pain behavior after peripheral nerve injury 40,41. Bäckryd et al. 30 found statistically significant IL-6 levels in patients with neuropathic pain. However, a cohort study with a larger sample size indicates that this cytokine shows no outstanding values one year later, where they found increases in CC and IL-8 34, which have also been related to neuropathic pain (42).
Although IL-6 is the most studied cytokine in relation to nerve pain, it is not the most significant value shown in the study by Chamessian et al. 35, where experimental patients experienced chronic pain due to injury from trauma to the stump nerve. Significantly higher levels were in TNF, IL-8, PCR, and growth factor-related proteins. In addition, it is important that the CRP has shown high values, because, despite being considered a marker in the early stages of inflammation, it is also considered a marker that allows the evaluation and monitoring of the development of inflammatory progression 27,43.
There is abundant evidence that certain proinflammatory cytokines such as IL-1β, IL-6, and TNF are involved in the process of pathological pain 21. This group of mediators has been analyzed by Teodorezyk-Injeyan et al. 31, who found higher values in the group of patients with chronic low back pain compared to the control group. In addition, isolated TNF also has shown significant differences between patients with chronic and acute low back pain. Lasselin et al. 33 also found significant values, but in this case behavioral therapy significantly reduced serum TNF concentrations. Both studies showed an association between pain and TNF, as their values decrease after the intervention or are lower in patients with no pain. It should be noted that TNF acts in various signaling pathways through its receptors in glial cells 44 and plays important roles in both inflammatory and neuropathic hyperalgesia 21.
Hysing et al. 32 also support the benefit of behavioral therapy in cytokines in the IL-8 group. In addition, in this study, 4 cytokines increased after treatment. This increase, such as the baseline value of chemokines expressed in acute phases, may be because, in contrast with rheumatic diseases, such as osteoarthritis (OA) or ankylosing spondylitis, chronic pain is not an inflammation in itself. In addition, in this study more than half of patients have associated problems, such as depression and/or anxiety, being associated processes that can contribute to the release of pro-inflammatory cytokines in patients with chronic pain, even in diseases not considered inflammatory.
Heffner et al. have also conducted a behavioral therapy study 38 showing a significant lower level of IL-6 after the intervention. One of the inclusion criteria was that participants had to have medical evidence of knee OA and pain for more than 6 months, so in this case inflammatory cytokines are associated with a properly inflammatory but chronic disease.
It has been observed in animal studies that localized inflammation in the DRG upregulates a number of pro-inflammatory cytokines, including IL-6, and induces an abnormal sympathetic response in the absence of peripheral nerve injury 20. In inflammatory diseases, such as OA, the role of the sympathetic nervous system may increase the patient's painful sensation due to peripheral sensitization caused by chemical stress associated with perivascular nerve growth 45.
As with neuropathic pain, IL-1β, IL-6, and TNF levels are also increased in patients with TMD from the Park et al study. 39. However, in these patients, despite having higher CRP values compared to the control group, the levels of the CRP are not as decisive or significant. TMD have been correlated with hyperalgesia in areas peripheral to injury 46, so the values of the analyzed inflammatory cytokines are understood due to their role in the process of pathological pain 21.
Finally, chronic pain has also been associated with increased and statistically significant levels of CRP in the studies of Sibille et al. 28, Feinberg et al. 29 and Skarpsno et al. 37. Given the large number of participants in the three studies and the relevance of CRP to pain mechanisms, because it is influenced by several chemokines, CRP can be considered as one of the most relevant markers and with more association to chronic pain, because CRP has been associated with generalized hyperalgesia, revealing an association between experimental pain tolerance and this protein 19.
TOLERANCE TO PAIN AND IM
Pain tolerance tests were used by Gerdle et al. 11, and the results were related to increased cytokines, including CC-type chemokines and IL-1β, which regulate and activate the pain signaling pathway, and, in particular, IL-1β increases the production of substance P in several neuronal and glial cells 47, this leads to an increase in peripheral sensitization and therefore decrease pain tolerance 6. This association leads us to analyze the study conducted by Schistad et al. 36, who show that hs-CRP levels are correlated with chronic pain patients, and were less tolerant to cold than the other groups. Considering the large sample size of this study, with 10,274 patients, and data reported by Gerdle et al. 11, an association is found between IM and patients with chronic pain, as well as with decreased pain tolerance.
INSOMNIA AND IM
Insomnia has been shown to be associated with IM due to increased IM when this clinical entity is stressed 13,14. For this reason, authors such as Skarpsno et al. 37, Heffner et al. 38 and Park et al. 39 analyzed the relationship that might be in their studies.
High levels of insomnia and high hs-CRP values were found to be associated with a higher likelihood of chronic pain 37. Park et al. 39 also related the worst sleep indicators to the higher pain and higher IM score groups. However, behavioral therapy was shown to be a technique that improves insomnia and significantly decreases IL-6 levels 38). Current evidence defines IL-6 as the most sensitive inflammatory mediator for sleep changes 48.
Some studies have shown that sleep restriction can induce an inflammatory response 48,49, which can contribute to the sensitization of the nociceptive system and thus increase susceptibility to the development of chronic pain 49.
As for the duration of sleep, the IM that shows the greatest association is the CRP. Sleep disorders is believed to have proximal effects in IL-6; but, as we discussed above, IL-6 induces CRP. Therefore, the increase in CRP could be due to a more persistent or severe sleep disturbance 48.
It is difficult to draw solid conclusions about sleep disorders by examining three articles, given the variety of differences between the methodology and inflammatory markers. But despite this, current evidence supports the extracted data, and treatment of insomnia has been found to reduce inflammation 50,51. Therefore, sleep should be a clinical entity to consider in the treatment of patients with chronic pain.
GENDER, INSOMNIA AND IM
In eight 11,28,29,34,36,39 of the thirteen studies included in this review, there are a larger number of women or only women were included. The fact that there are more women could be because the results indicate that women are more likely to have chronic pain due to factors such as menstrual cycle, temporal summation of pain, endogenous inhibitory mechanisms of pain, and gender beliefs and expectations 52. Moreover, the past individual medical history influences the painful response in women rather than men, in short, other psychosocial factors could contribute to differences in pain sensitivity between men and women. Sibille et al. 28 found that chronic pain in women was significantly higher than in men, compared with the control group.
Sex differences, predominantly female, exist in sleep quality, duration, latency, and insomnia 53. However, complaints of insomnia and daytime sleepiness are quite more frequent in women, with 58% compared with 42% in men 54. However, Heffner et al. 38 showed that their male patients had more insomnia than women, although this difference was not significant. Despite this, it appears that women may be more vulnerable to the effects of sleep disorder and show higher increases in IL-6 and CRP 55.
ANXIETY, DEPRESSION, AND IM
A stimulation of the immune system and increased secretion of immune parameters such as IL-6 and TNF are associated with a negative mood 56,57. Increased cytokine concentrations and insomnia have also been associated with the risk of depression 58,59,60.
It has been proposed that the factors generating stress initiate a cascade of biochemical events that also involves the participation of IM of the immune system, cooperating all of them in the appearance and expression of depression 61.
This review suggests that behavioral therapy reduces TNF levels, but that in only patients with low inflammatory status it may reduce unhappiness 33. It also produced improvements in inflammatory levels in patients with anxiety and depression 32. In spite of this, the sample in both studies is small, so the claims should be taken with caution.
Sibille et al. 28 show that chronic pain did not correlate depression and anxiety. Schistad et al. 36 found no association with inflammatory levels using the same assessment scale. Only Teodorezyk-Injeyan et al. found an association between anxiety and depression and IM 31, therefore, although the evidence has found an association between increased IM values and the risk of such clinical symptoms, the data are inconclusive.
It was concluded that there are significantly higher statistical values in patients with chronic pain than in the control group in some protein or inflammatory marker analyzed. Despite the variability of the mediators analyzed, CRP has been the most analyzed protein in all articles, followed by: TNF, IL-6, IL-8 and IL-1β; ordered from highest to lowest number of occurrences in the articles. Early diagnosis would help improve treatment.
In the same way it is shown association of suffering chronic pain with increased values of the IM, which in turn decrease the tolerance to the pain. It is also related to insomnia, with a higher prevalence in women and obesity. Contrary to the previous evidence, no significant association with anxiety and depression has been found in these articles.
CONCLUSIONS
Therefore, the measurement of CRP and proinflammatory cytokines could lead to an advance in the assessment and follow-up of the patient with chronic pain. Due to the current difficulty in making these measurements in clinical practice, healthcare professionals must understand the pain and all associated mechanisms that should be considered for proper treatment and management of the pain.
BIBLIOGRAFÍA
1. Wall PD, McMahon SB. Microneuronography and Its Relation to Perceived Sensation. A Critical Review. Pain. 1985;21(3):209-29. DOI: 10.1016/0304-3959(85)90086-7. [ Links ]
2. Raja S, Carr D, Cohen M, Finnerup N, Flor H, Gibson S, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976-82. DOI: 10.1097/j.pain.0000000000001939. [ Links ]
3. Backonja MM. Defining Neuropathic Pain. Anesth Analg. 2003;97(3):785-90. DOI: 10.1213/01.ane.0000062826.70846.8d. [ Links ]
4. National Institute for Health and Clinical Excellence. Neuropathic Pain: The Pharmacological Management of Neuropathic Pain in Adults in Non-Specialist Settings. Clinical Guildeline 96. London: NICE; 2010. [ Links ]
5. Moseley GL. A Pain Neuromatrix Approach to Patients with Chronic Pain. Man Ther. 2003;8(3):130-40. DOI: 10.1016/s1356-689x(03)00051-1. [ Links ]
6. García-Andreu J. Manejo Básico Del Dolor Agudo y Crónico. Anest Mex. 2017;29(1). [ Links ]
7. Zegarra JW. Bases Fisiopatológicas Del Dolor. Acta Med Per. 2007;24(2):35-8. [ Links ]
8. Jussila L, Paananen M, Näyhä S, Taimela S, Tammelin T, Auvinen J, et al. Psychosocial and Lifestyle Correlates of Musculoskeletal Pain Patterns in Adolescence: A 2-Year Follow-up Study. Eur J Pain. 2014;18(1):139-46. DOI: 10.1002/j.1532-2149.2013.00353.x. [ Links ]
9. Langley PC, Ruiz-Iban MA, Molina JT, De Andres J, Castellón JR, et al. The Prevalence, Correlates and Treatment of Pain in Spain. J Med Econ. 2011;14(3):367-80. DOI: 10.3111/13696998.2011.583303. [ Links ]
10. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of Chronic Pain in Europe: Prevalence, Impact on Daily Life, and Treatment. Eur J Pain. 2006;10(4):287-333. DOI: 10.1016/j.ejpain.2005.06.009. [ Links ]
11. Gerdle B, Ghafouri B, Ghafouri N, Bäckryd E, Gordh T. Signs of Ongoing Inflammation in Female Patients with Chronic Widespread Pain: A Multivariate, Explorative, Cross-Sectional Study of Blood Samples. Medicine. 2017;96(9): e6130. DOI: 10.1097/MD.0000000000006130. [ Links ]
12. Brosschot JF. Cognitive-Emotional Sensitization and Somatic Health Complaints. Scand J Psychol. 2002;43(2):113-21. DOI: 10.1111/1467-9450.00276. [ Links ]
13. Allen KD, Renner JB, Devellis B, Helmick CG, Jordan JM. Osteoarthritis and Sleep: The Johnston Country Osteoarthritis Project. J Rheumatol. 2008;35(6):1102-107. [ Links ]
14. Burgos I, Richter L, Klein T, Fiebich B, Feige B, Lieb K, et al. Increased Nocturnal Interleukin-6 Excretion in Patients with Primary Insomnia: A Pilot Study. Brain Behav. Immun. 2006;20(3):246-53. DOI: 10.1016/j.bbi.2005.06.007. [ Links ]
15. Prather AA, Puterman E, Epel ES, Dhabhar FS. Poor Sleep Quality Potentiates Stress-Induced Cytokine Reactivity in Postmenopausal Women with High Visceral Abdominal Adiposity. Brain. Behav. Immun. 2014;35:155-62. DOI: 10.1016/j.bbi.2013.09.010. [ Links ]
16. Blyth FM, March LM, Brnabic AJ, Cousins MJ. Chronic Pain and Frequent Use of Health Care. Pain. 2004;111(1-2):51-8. DOI: 10.1016/j.pain.2004.05.020. [ Links ]
17. Chatkoff DK, Leonard MT, Maier KJ. Pain Catastrophizing Differs Between and Within West Haven-Yale Multidimensonal Pain Inventory (MPI) Pain Adjustment Classifications. Clin J Pain. 2015;31(4):349-54. DOI: 10.1097/AJP.0000000000000117. [ Links ]
18. Kaunisto MA, Jokela R, Tallgren M, Kambur O, Tikkanen E, Tasmuth T, et al. Pain in 1,000 Women Treated for Breast Cancer: A Prospective Study of Pain Sensitivity and Postoperative Pain. Anesthesiology. 2013;119(6):1410-21. DOI: 10.1097/ALN.0000000000000012. [ Links ]
19. Afari N, Mostoufi S, Noonan C, Poeschla B, Succop A, Chopko L, et al. C-Reactive Protein and Pain Sensitivity: Findings from Female Twins. Ann Behav Med. 2011;42(2):277-83. DOI: 10.1007/s12160-011-9297-6. [ Links ]
20. Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142(3):809-22. DOI: 10.1016/j.neuroscience.2006.06.045. [ Links ]
21. Zhang JM, Jianxiong A. Cytokines, Inflammation and Pain. Int Anesthesiol Clin. 2007;45(2):27-37. DOI: 10.1097/AIA.0b013e318034194e. [ Links ]
22. Jabs WJ, Lögering BA, Gerke P, Kreft B, Wolber EM, Flinger MHF, et al. The Kidney as a Second Site of Human C-Reactive Protein Formation in Vivo. Eur J Immunol. 2003;33(1):152-61. DOI: 10.1002/immu.200390018. [ Links ]
23. Ramage L, Proudfoot L, Guy K. Expression of C-Reative Protein in Human Lung Epithelial Cells and Upregulation by Cytokines and Carbon Particles. Inhal Toxicol. 2004;16(9):607-13. [ Links ]
24. Yasojima K, Schwab C, McGeer EG, McGeer PL. Human Neurons Generate C-Reactive Protein and Amyloid P: Upregulation in Alzheimer's Disease. Brain Res. 2000;887(1):80-9. DOI: 10.1016/s0006-8993(00)02970-x. [ Links ]
25. Calabro P, Chang DW, Willerson JT, Yeh ET. Release of C-Reactive Protein in Response to Inflammatory Cytokines by Human Adipocytes: Linking Obesity to Vascular Inflammation. J Am Coll Cardiol. 2005;46(6):1112-3. DOI: 10.1016/j.jacc.2005.06.017. [ Links ]
26. Kuta AE, Baum LL. C-Reactive Protein Is Produced by a Small Number of Normal Human Peripheral Blood Lymphocytes. J Exp Med. 1986;164(1):321-6. DOI: 10.1084/jem.164.1.321. [ Links ]
27. Du Clos TW, Mold C. C-Reactive Protein: An Activator of Innate Immunity and a Modulator of Adaptive Immunity. Immun Res. 2004;30(3):261-77. DOI: 10.1385/IR:30:3:261. [ Links ]
28. Sibille KT, Steingrímsdóttir OA, Fillingim RB, Stubhaug A, Schirmer H, Chen H, et al. Investigating the Burden of Chronic Pain: An Inflammatory and Metabolic Composite. Pain Res. 2016;2016:7657329. DOI: 10.1155/2016/7657329. [ Links ]
29. Feinberg T, Sambamoorthi U, Lilly C, Innes KK. Potential Mediators between Fibromyalgia and C-Reactive Protein: Results from a Large U.S. Community Survey. BMC Musculoskelet. Disord. 2017;18(1):294. DOI: 10.1186/s12891-017-1641-y. [ Links ]
30. Bäckryd E, Ghafouri B, Larsson B, Gerdle B. Plasma Pro-Inflammatory Markers in Chronic Neuropathic Pain: A Multivariate, Comparative, Cross-Sectional Pilot Study. Scand J Pain. 2016;10(1):1-5. DOI: 10.1016/j.sjpain.2015.06.006. [ Links ]
31. Teodorczyk-Injeyan JA, Triano JJ, Injeyan HS. Nonspecific Low Back Pain: Inflammatory Profiles of Patients With Acute and Chronic Pain Clin J Pain. 2019;35(10):818-25. DOI: 10.1097/AJP.0000000000000745. [ Links ]
32. Hysing EB, Smith L, Thulin M, Karlsten R, Bothelius K, Gordh T. Detection of Systemic Inflammation in Severely Impaired Chronic Pain Patients and Effects of a Multimodal Pain Rehabilitation Program. Scand J Pain. 2019;19(2):235-44. DOI: 10.1515/sjpain-2018-0340. [ Links ]
33. Lasselin J, Kemani MK, Kanstrup M, Olsson GL, Axelsson J, Andreasson A, et al. Low-Grade Inflammation May Moderate the Effect of Behavioral Treatment for Chronic Pain in Adults. J Behav Med. 2016;39(5):916-24. DOI: 10.1007/s10865-016-9769-z. [ Links ]
34. Bäckryd E, Lind AL, Thulin M, Larsson A, Gerdle B, Gordh T. High Levels of Cerebrospinal Fluid Chemokines Point to the Presence of Neuroinflammation in Peripheral Neuropathic Pain: A Cross-Sectional Study of 2 Cohorts of Patients Compared with Healthy Controls. Pain. 2017;158(12):2487-95. DOI: 10.1097/j.pain.0000000000001061. [ Links ]
35. Chamessian A, Van de Ven T, Buchheit T, Hsia HL, McDuffie M, Gamazon ER, et al. Differential Expression of Systemic Inflammatory Mediators in Amputees with Chronic Residual Limb Pain. Pain. 2017;158(1):68-74. DOI: 10.1097/j.pain.0000000000000728. [ Links ]
36. Schistad EI, Stubhaug A, Furberg AS, Engdahl BL, Nielsen CS. C-Reactive Protein and Cold-Pressor Tolerance in the General Population: The Tromsø Study. Pain. 2017;158(7):1280-8. DOI: 10.1097/j.pain.0000000000000912. [ Links ]
37. Skarpsno ES, Mork PJ, Nilsen TI, Steingrímsdóttir ÓA, Zwart JA, Nilsen KB. The Interplay between Sleeplessness and High-Sensitivity C-Reactive Protein on Risk of Chronic Musculoskeletal Pain: Longitudinal Data from the Tromsø Study. Sleep. 2019;42(9):127. DOI: 10.1093/sleep/zsz127. [ Links ]
38. Heffner KL, France CR, Ashrafioun L, Quiñones M, Walsh P, Maloney MD, et al. Clinical Pain-Related Outcomes and Inflammatory Cytokine Response to Pain Following Insomnia Improvement in Adults With Knee Osteoarthritis. Clin J Pain. 2018;34(12):1133-40. DOI: 10.1097/AJP.0000000000000644. [ Links ]
39. Park J, Chung J. Inflammatory Cytokines and Sleep Disturbance in Patients with Temporomandibular Disorders. Oral Facial Pain Headache. 2016;30(1):27-33. DOI: 10.11607/ofph.1367. [ Links ]
40. De Leo JA, Colburn RW. The Role of Cytokines in Nociception and Chronic Pain. In: Weinstein JN, Gordon SL, Editors. Low Back Pain: A Scientific and Clinical Overview. Illinois: American Academy of Orthopaedic Surgeons; 1995. p. 163-85. [ Links ]
41. Ramer MS, Murphy PG, Richardson PM, Bisby MA. Spinal Nerve Lesion-Induced Mechanoallodynia and Adrenergic Sprouting in Sensory Ganglia Are Attenuated in Interleukin-6 Knockout Mice. Pain. 1998;78(2):115-21. DOI: 10.1016/s0304-3959(98)00121-3. [ Links ]
42. Old EA, Clark AK, Malcangio M. The Role of Glia in the Spinal Cord in Neuropathic and Inflammatory Pain. Handb Exp Pharmacol. 2015;227:145-70. DOI: 10.1007/978-3-662-46450-2_8. [ Links ]
43. Yao ZY, Zhang Y, Wu H. Regulation of C-reactive protein conformation in inflammation. Inflammation Research. 2019;68(10):815-23. DOI: 10.1007/s00011-019-01269-1. [ Links ]
44. Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. Immunocytochemical Analysis of Tumor Necrosis Factor and Its Receptors in Parkinson's Disease. Neuroscience Letters. 1994;172(1-2):151-4. DOI: 10.1016/0304-3940(94)90684-x. [ Links ]
45. Grässel S, Muschter D. Peripheral Nerve Fibers and Their Neurotransmitters in Osteoarthritis Pathology. Int J Mol Sci. 2017;18(5):931. DOI: 10.3390/ijms18050931. [ Links ]
46. Smith MT, Wickwire EM, Grace EG, Edwards RR, Buenaver LF, Peterson S, et al. Sleep Disorders and Their Association with Laboratory Pain Sensitivity in Temporomandibular Join Disorder. Sleep. 2009;32(6):779-90. DOI: 10.1093/sleep/32.6.779. [ Links ]
47. Jeanjean AP, Moussaoui SM, Maloteaux JM, Laduron PM. Interleukin-1 Beta Induces Long-Term Increase of Axonally Transported Opiate Receptors and Substance P. Neuroscience. 1995;68(1):151-7. DOI: 10.1016/0306-4522(95)00106-s. [ Links ]
48. Irwin MR, Olmstead R, Judith E, Carroll JE. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review an Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol Psychiatry. 2016;80(1):40-52. DOI: 10.1016/j.biopsych.2015.05.014. [ Links ]
49. Haack M, Sanchez E, Mullington JM. Elevated Inflammatory Markers in Response to Prolonged Sleep Restriction Are Associated with Increased Pain Experience in Healthy Volunteers. Sleep. 2007;30(9):1145-52. DOI: 10.1093/sleep/30.9.1145. [ Links ]
50. Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Breen EC, Witarama T, et al. Cognitive Behavioral Therapy vs. Tai Chi for Late Life Insomnia and Inflammatory Risk: A Randomized Controlled Comparative Efficacy Trial. Sleep. 2014;37(9):1543-52. DOI: 10.5665/sleep.4008. [ Links ]
51. Irwin MR, Olmstead R, Breen EC, Witarama T, Carrillo C, Sadeghi N, et al. Cognitive Behavioral Therapy and Tai Chi Reverse Cellular and Genomic Markers of Inflammation in Late Life Insomnia: A Randomized Controlled Trial. Biol Psychiatry. 2015;78(10):721-9. DOI: 10.1016/j.biopsych.2015.01.010. [ Links ]
52. Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choinire M. A Systematic Literature Review of 10 Years of Research on Sex/Gender and Pain Perception - Part 2: Do Biopsychosocial Factors Alter Pain Sensitivity Differently in Women and Men? Pain. 2012;153(3):619-35. DOI: 10.1016/j.pain.2011.11.026. [ Links ]
53. Monica P, Christine L. Exploring Sex and Gender Differences in Sleep Health: A Society for Women's Health Research Report. J Womens Health. 2014;23(7):553-62. DOI: 10.1089/jwh.2014.4816. [ Links ]
54. Regal AR, Amigo MC, Cebrian E. El Sueño y Mujer. Rev Neurology. 2009;49:376-82. DOI: 10.33588/rn.4907.2009041. [ Links ]
55. Prather AA, Epel ES, Cohen BE, Neylan TC, Whooley MA. Gender Differences in the Prospective Associations of Self-Reported Sleep Quality with Biomarkers of Systemic Inflammation and Coagulation: Findings from the Heart and Soul Study. J Psychiatr Res. 2013;47(9):1228-35. DOI: 10.1016/j.jpsychires.2013.05.004. [ Links ]
56. Dinan TG. Inflammatory Markers in Depression. Curr Opin Psychiatry. 2009;22(1):32-6. DOI: 10.1097/YCO.0b013e328315a561. [ Links ]
57. Rief W, Pilger F, Ihle D, Bosmans E, Egyed B, Maes M. Immunological Differences between Patients with Major Depression and Somatization Syndrome. Psychiatry Res. 2001;105(3):165-74. DOI: 10.1016/s0165-1781(01)00338-9. [ Links ]
58. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A Meta-Analysis of Cytokines in Major Depression. Biol Psychiatry. 2010;67(5):446-57. DOI: 10.1016/j.biopsych.2009.09.033. [ Links ]
59. Howren MB, Lamkin DM, Suls J. Associations of Depression with C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosom Med. 2009;71(2):171-86. DOI: 10.1097/PSY.0b013e3181907c1b. [ Links ]
60. Almeida OP, Alfonso H, Yeap BB, Hankey G, Flicker L. Complaints of Difficulty to Fall Asleep Increase the Risk of Depression in Later Life: The Health in Men Study. J Affect Disord. 2011;134(1-3):208-16. DOI: 10.1016/j.jad.2011.05.045. [ Links ]
61. Anisman H. Cascading Effects of Stressors and Inflammatory Immune System Activation: Implications for Major Depressive Disorder. J Psychiatry Neurosci. 2009;34(1):4-20. [ Links ]
Received: August 19, 2020; Accepted: December 02, 2020











 texto em
texto em