Anales del Sistema Sanitario de Navarra
ISSN 1137-6627
18--2024
https://dx.doi.org/10.23938/assn.1060
Original Articles
Series of patients operated for masses and cysts in the heart in a Spanish general hospital: a 20-year experience
1.University of Navarra. School of Medicine. Pamplona. Navarre. Spain
2.Clínica Universidad de Navarra. Department of Cardiology and Cardiac Surgery. Pamplona. Navarre. Spain
Background.
Masses and cysts in the heart are well-known entities, but their low prevalence and non-specific symptoms makes the diagnosis difficult. We aimed to characterize the features of these entities in our environment.
Methods.
We carried out a search of patients who underwent surgery for tumors and cysts in the heart between 2002 and 2022 in the registry of the Department of Cardiology and Cardiac Surgery of Clínica Universidad de Navarra (Pamplona, Spain). Sociodemographic, clinical, histological, and surgical variables were collected.
Results.
We identified 13 patients; mean age was 63.08 ± 15.17 years, 76.92% were female and 92.31% had at least one cardiovascular risk factor, e.g., BMI ≥ 25 kg/m2 and high blood pressure (61.54% and 53.85%, respectively). The most common type of cardiac tumors were myxomas (69.23%). Around half (46.15%) were incidental; the most frequent symptom was dyspnea (53.85%); 30.77% of the patients were asymptomatic. The most commonly used imaging technique for the diagnosis was transthoracic Doppler echocardiography (69.23%). The agreement between the mean diameters before and after surgery was very high (ICC = 0.807, 95%CI: 0.450-0.943).
Conclusions.
We describe the features of masses and cysts in the heart (77% female patients) and provide information scarcely available in the literature, e.g., the most frequent cardiovascular risk factors for this population. A case of cardiac leiomyosarcoma and a case of intimal sarcoma of the pulmonary trunk are described, two extremely rare tumors for which there are few described cases.
Keywords: Cardiology; Cardiac Surgery; Cardiovascular Diseases; Heart Neoplasms; Myxoma
INTRODUCTION
Despite being widely known entities by the healthcare community, the prevalence of cardiac masses is less than 0.3%1, with a cumulative incidence of 13.8 cases per million people per year in the case of primary tumor2. The differential diagnosis of these neoplasms includes other cardiac masses, e.g., vegetations, thrombi, or myocardial hypertrophy3, as well as other entities such as vascular neoplasms or intrapericardial cysts.
Cardiac tumors may be primary or secondary (metastases)4, , the latter being up to 132 times more frequent than the former5. Primary tumors can further be classified as benign (75%)6, or malignant7. As for benign primary cardiac tumors in adults, most are myxomas and are usually found in the left atrium9, while the most frequently found benign primary tumor in children is rhabdomyoma. Of the 25% malignant ones, the most common are sarcomas and lymphomas8.
Cardiac masses affect people mainly between the ages of 30 and 6010 and some, such as myxomas, are more commonly found in women3. The histopathology of cardiac masses is very diverse. For example, myxomas are gelatinous structures formed by stellate or polygonal spindle cells surrounded by a stroma rich in glycosaminoglycans. These cells are arranged in rings around amorphous capillaries and the tumor surface is surrounded by endothelium11,12.
The clinical presentation of cardiac masses is determined by their size, anatomical location, and mobility, rather than by their histopathology7,11. Therefore, symptoms are usually non-specific (chest pain, syncope, murmurs, or arrhythmias)3, , which makes diagnosis difficult as it overlaps with typical presentations of other cardiovascular conditions. However, a high proportion of patients are asymptomatic at diagnosis, with the tumor being found incidentally through imaging tests performed for another reason3. The improvement in the quality and increased use of cardiac imaging techniques in recent years have contributed to increasing the number of diagnoses that are made incidentally.13.
According to current recommendations for the diagnosis of cardiac masses, echocardiography is the imaging technique of choice; the study can be completed with other imaging modalities such as computed tomography (CT) or magnetic resonance (MRI)8. Despite the great information that these tests provide, the definitive diagnosis must be histological11.
The treatment of cardiac masses is based on surgical excision regardless of their size given the risk of causing lethal complications3,11.Minimally invasive surgery has allowed patients to achieve increasingly earlier functional recovery14. In general, cardiac masses have a good prognosis8,11 and low risk of recurrence (2% in the largest series published to date)15.
The low prevalence and non-specific symptoms make it difficult to include cardiac masses in the clinical differential diagnosis. Furthermore, the literature focuses primarily on myxomas, and data on other less common cardiac masses are scarce. The objective of this study was to carry out an exhaustive characterization of the symptoms experienced by patients diagnosed with cardiac masses and cysts in our environment, aiming to obtain further data for future diagnoses and provide patients with optimal clinical care.
MATERIAL AND METHODS
Descriptive, retrospective, single-center study. A search was carried out to identify patients who underwent surgery for cardiac tumors and cysts between December 2002 and December 2022 (twenty years) in the database of the Department of Cardiology and Cardiac Surgery of Clínica Universidad de Navarra (Pamplona, Spain).
The following variables were extracted from the medical records:
- Sociodemographic: year of diagnosis, sex, and age at diagnosis;
- Clinical: cardiovascular risk factors (CVRF) such as body mass index (BMI, kg/m2) - categorized as normal weight (18.50-24.99), overweight (25.00-29.99), type I obesity (30.00-34.99), and type II obesity (35.00-39.99) -, arterial hypertension, diabetes mellitus, dyslipidemia, hyperuricemia, and tobacco use (non-smoker, former smoker, active smoker); personal history (cardiovascular, respiratory, oncological disease, previous cardiac surgery), and family history;
- Cardiac: symptoms, diagnostic imaging technique, type of finding (incidental, due to symptoms), and anatomical location;
- Preoperative: mean pre-surgical diameter (mm), imaging technique used for determining the size of the mass, and mean diameter measured after surgical removal of the mass;
- Anatomopathological: type of cardiac mass (myxoma, leiomyosarcoma, intimal sarcoma, intrapericardial cyst, thrombus).
Likewise, the pathological reports and description of the surgical interventions recorded by the surgical team were consulted.
The specific time of the checkup (in days after surgery), symptoms, imaging techniques used, and data on recurrence and exitus were recorded during the first postoperative checkup (while in the hospital) and in the first checkup following hospital discharge.
This study was conducted in compliance with the Declaration of Helsinki and approved by the Research Ethics Committee of the University of Navarra (project 2023.140). Patient information was coded to protect their identity.
Statistical analysis. The Shapiro-Wilk test, along with the evaluation of skewness and kurtosis, was used to determine whether continuous variables followed the normal distribution. Variables following a normal distribution are presented using arithmetic means (-x) and standard deviations (SD); otherwise, with medians (P50) and interquartile ranges (IQR). Qualitative variables were presented using frequencies and percentages. The Mann-Whitney U test was used to compare mean postoperative diameters of the masses found in the cardiac chambers according to their anatomical location. Agreements between mean diameters before and after surgery were evaluated using the intraclass correlation coefficient (ICC), together with its 95% confidence interval (95%CI). p-values were two-tailed and statistical significance was set at p <0.05. The analyses were performed with STATA version 15.1 (Stata Corp, College Station, TX, USA) and GraphPad Prism version 9.5.1 (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
Thirteen patients were identified in the search, all of whom were included in the study. In this sample, there was a higher percentage of female patients (76.92%) and the average age was 63.08 years (range: 30-86). Table 1 shows the baseline characteristics of the study patients based on the type of cardiac mass.
Tabla 1. Características basales de los pacientes seleccionados.
| Variables | Type of cardiac mass diagnosed | Total n = 13 | ||||
|---|---|---|---|---|---|---|
| Myxoma n = 9 | Leiomiosarcoma n = 1 | Intimal sarcoma with associated thrombosis n = 1 | Intrapericardial cyst n = 1 | Thrombus n = 1 | ||
| Sex (female), n (%) | 8 (88,89) | 1 (11,11) | 0 | 1 (11,11) | 0 | 10 (76,92) |
| Age (years), mean (SD) | 63,22 (7,48) | 64 | 74 | 64 | 49 | 63,08 (15,17) |
| Cardiovascular risk factors, n (%) | ||||||
| BMI (kg/m2), mean (SD) | 26,36 (4,23) | 26,5 | 30,1 | 36 | 24,9 | 27,36 (5,50) |
| HTN | 5 (55,56) | 0 | 1 (100) | 1 (100) | 0 | 7 (53,85) |
| DM | 0 | 0 | 1 (100) | 1 (100) | 0 | 2 (15,38) |
| Dyslipidemia | 5 (55,56) | 1 (100) | 0 | 0 | 0 | 6 (46,15) |
| Hyperuricemia | 2 (22,22) | 0 | 0 | 0 | 0 | 2 (15,38) |
| Tobacco use | ||||||
| Non-smoker | 6 (66,67) | 1 (100) | 1 (100) | 1 (100) | 0 | 9 (69,23) |
| Former smoker | 2 (22,22) | 0 | 0 | 0 | 1 (100) | 3 (23,08) |
| Active smoker | 1 (11,11) | 0 | 0 | 0 | 0 | 1 (7,69) |
| Personal history, n (%) | ||||||
| Cardiovascular | 2 (22,22) | 0 | 0 | 0 | 1 (100) | 3 (23,08) |
| Respiratory | 1 (11,11) | 0 | 1 (100) | 1 (100) | 0 | 3 (23,08) |
| Oncological | 2 (22,22) | 0 | 0 | 0 | 0 | 2 (15,38) |
| Previous cardiac surgery | 0 | 0 | 0 | 0 | 1 (100) | 1 (7,69) |
| Family history, n (%) | ||||||
| Cardiovascular | 2 (22,22) | 0 | 0 | 0 | 1 (100) | 3 (23,08) |
| Symptoms,, n (%) | ||||||
| Asymptomatic | 3 (33,33) | 0 | 0 | 0 | 1 (100) | 4 (30,77) |
| Dyspnea | 4 (44,44) | 1 (100) | 1 (100) | 1 (100) | 0 | 7 (53,85) |
| Palpitations at rest | 2 (22,22) | 0 | 0 | 0 | 0 | 2 (15,38) |
| Malaise | 1 (11,11) | 0 | 0 | 0 | 0 | 1 (7,69) |
| Asthenia | 2 (22,22) | 0 | 0 | 0 | 0 | 2 (15,38) |
| Presyncope | 2 (22,22) | 0 | 0 | 0 | 0 | 2 (15,38) |
| Epigastric pain | 1 (11,11) | 0 | 0 | 0 | 0 | 1 (7,69) |
| Thoracic pain not related to exercise | 1 (11,11) | 0 | 0 | 0 | 0 | 1 (7,69) |
| Lower limb edema | 1 (11,11) | 0 | 0 | 1 (100) | 0 | 2 (15,38) |
| Upper limb edema | 1 (11,11) | 0 | 0 | 0 | 0 | 1 (7,69) |
| Diagnostic imaging technique, n (%) | ||||||
| TTE Doppler color | 8 (88,89) | 0 | 0 | 0 | 1 (100) | 9 (69,23) |
| Thoracic CTA | 0 | 0 | 1 (100) | 0 | 0 | 1 (7,69) |
| Thoracic CT | 1 (11,11) | 0 | 0 | 0 | 0 | 1 (7,69) |
| Thoracic CT (IV contrast) | 0 | 1 (100) | 0 | 1 (100) | 0 | 2 (15,38) |
| Type of finding, n (%) | ||||||
| Incidental | 4 (44,44) | 0 | 0 | 1 (100) | 1 (100) | 6 (46,15) |
| Due to symptoms | 5 (55,56) | 1 (100) | 1 (100) | 0 | 0 | 7 (53,85) |
| Anatomical location, n (%) | ||||||
| Right atrium | 1 (11,11) | 0 | 0 | 0 | 1 (100) | 2 (15,38) |
| Left atrium | 8 (88,89) | 1 (100) | 0 | 0 | 0 | 9 (69,23) |
| Right predominance | 0 | 0 | 0 | 1 (100) | 0 | 1 (7,69) |
| Pulmonary trunk | 0 | 0 | 1 (100) | 0 | 0 | 1 (7,69) |
| Mean pre-surgical diameter (mm), mean (SD) | 34,31 (13,85) | * | 37 | 79 | 28,5 | 37,78 (17,65) |
| Measurement technique, n (%) | ||||||
| TTE Doppler color | 6 (66,67) | 1 (100) | 0 | 0 | 1 (100) | 8 (61,54) |
| TEE Doppler color | 2 (22,22) | 0 | 0 | 0 | 0 | 2 (15,38) |
| Thoracic CTA | 0 | 0 | 1 (100) | 0 | 0 | 1 (7,69) |
| Thoracic CT (IV contrast) | 0 | 0 | 0 | 1 (100) | 0 | 1 (7,69) |
| Cardiac MRI | 1 (11,11) | 0 | 0 | 0 | 0 | 1 (7,69) |
| Mean post-surgical diameter (mm), mean (SD) | 38,00 (17,15) | ** | 40 | *** | 20 | 36,55 (16,31) |
BMI: body mass index; CT: computed tomography; CTA: computed tomography angiography; DM: diabetes mellitus; HTN: arterial hypertension; IV: intravenous; MRI: magnetic resonance imaging; SD: standard deviation; TEE: transesophageal echocardiogram; TTE: transthoracic echocardiogram;
*:not measured;
**:very infiltrative mass, impossible to resect as a whole and measure it accurately;
***:not measured because it was a liquid collection.
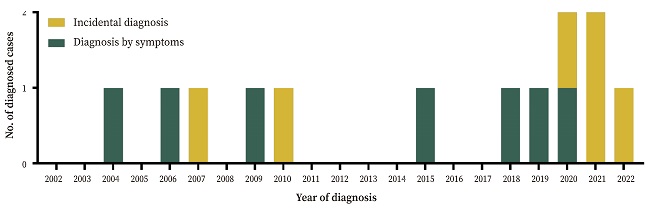
Year of diagnosis
The cumulative incidence was non-homogeneous throughout the study period. Halfway through the study (December 2012), only five cases (38.56%) had been diagnosed. Almost half of the patients (53.85%, n = 7) were diagnosed in the first sixteen years (until December 2018).
The cumulative incidence of incidental diagnoses also increased over the years: in the first 10 years, they accounted for 28.57% of diagnoses compared to 66.66% in the following 10 years (and 100% in the last two years) (Fig. 1).
Type of cardiac mass and anatomical location
The most common type of cardiac mass was the myxoma (69.23%, n = 9), most (88.89%, n = 8) found in the left atrium. Only one was located in the right atrium. In one case, the mass was implanted in the fossa ovalis, protruding into the left ventricle in diastole, causing mitral regurgitation. The histological report stated that in addition to the background of mucopolysaccharides (present in all), two cases contained foci of hemorrhagic necrosis, one associated bacterial colonies, and one had a notable vascular component with poorly formed vessels.
One patient presented a leiomyosarcoma in the left atrium, although it completely occupied the lumen of both right pulmonary veins and extended to the lobar and segmental veins. The surgical team reported that it was a very hard, infiltrating whitish tumor, which made it impossible to resect as a whole nor measure it. The histological report indicated that the mass presented great heterogeneity with microcalcifications and abundant inflammatory infiltrates.
A diagnosis of malignant intimal sarcoma with associated thrombosis was made in one patient. The tumor was located in the pulmonary trunk, extending to the right pulmonary artery and to the left one to a lesser extent. The mass showed muscular differentiation and the fluorescence in situ hybridization (FISH) study revealed an amplification of the mdm2 gene. After resection, adjuvant treatment consisting of radiotherapy (IMRT/VMAT technique) and chemotherapy (temozolomide) was offered, but the patient was not treated in our hospital.
An extracavitary intrapericardial cyst with right predominance was found in another patient. It compressed the right atrium and ventricle, displacing the right coronary artery anteriorly and extending along the inferior surface of the heart. Initially, a differential diagnosis was proposed between encapsulated pericardial effusion and chronic hemopericardium. Once liquid was drained, it was seen that it contained a fund of partially lysed red blood cells and abundant necrotic content corresponding to coagulation necrosis without inflammatory infiltrate or viable cells. It was concluded that the sample corresponded to an old hemopericardium.
Finally, one patient presented a fibrin thrombus in the right atrium anchored in Chiari’s network.
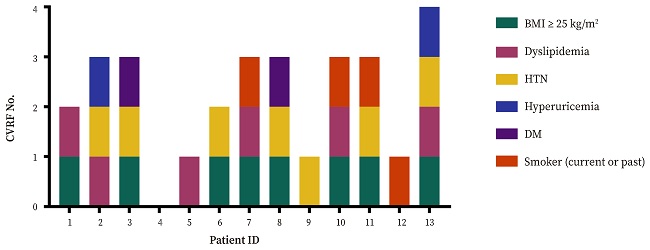
Cardiovascular risk factors
Only one patient (7.69%) had no cardiovascular risk factors, 23.08% (n = 3) had one, 15.38% (n = 2) had two, almost half (46.15%, n = 6), three and one patient had up to four.
Overweight/obesity was the most prevalent cardiovascular risk factor (61.54%, n = 8); five patients having overweight (38.46%), one had type I obesity (7.69%), and two type II obesity (15.38%). The next most frequent cardiovascular risk factors were arterial hypertension (53.85%, n = 7) and dyslipidemia (46.15%, n = 6) (Fig. 2).
Personal and family history
Three patients had a previous history of respiratory disease (bronchial asthma, pulmonary tuberculosis, and obstructive sleep apnea-hypopnea syndrome), two had a cardiovascular history (atrial fibrillation, Horton’s arteritis, and bilateral varicose syndrome), and two had a history of oncological disease (basal cell carcinoma in the nose, infiltrating pituitary macroadenoma secondary to acromegaly). The Cardiac Surgery team had previously operated one study patient for the closure of an ostium secundum atrial septal defect, suffering the patient a stroke in the postoperative period. However, this procedure was done three years before the diagnosis of the cardiac mass - a fibrin thrombus - and was not in contact with the closure of the previous atrial septal defect.
As for the family history, the maternal grandmother of one patient suffered from arrhythmia, the mother of another patient had diabetes mellitus and the father had diabetes mellitus and arterial hypertension. The mother of a third patient suffered from arterial hypertension and diabetes mellitus, and had suffered an acute myocardial infarction, her father had a stroke and myocardial infarction, and three of her siblings had had a stroke. None of the study patients reported a family history of cardiac masses.
Symptoms and type of tumor
The most prevalent symptom was dyspnea, present in around half of the patients (53.85%, n = 7), followed by palpitations at rest, asthenia, presyncope, and lower limb edema, each experienced by two patients.
In almost half of the cases (46.15%, n = 6) the discovery of the cardiac mass was incidental while performing a transthoracic color Doppler echocardiogram. Four patients (30.77%) had no symptoms. In one patient, the mass was identified in a checkup of the closure of a previous atrial septal defect; in another, when studying an already known heart murmur detected during a checkup of an urinary tract infection; in a third patient when evaluating previous atrial fibrillation and arterial hypertension in the cardiology clinic during the preoperative period of an inguinal hernia correction; and in the fourth, while attending the cardiology clinic on their initiative for a checkup. In two patients, the mass was found in a thoracic CT scan while performing the test for a different pathology upon hospital admission for SARS-CoV-2 pneumonia and with suspicion of asthmatic decompensation (the test was performed using intravenous contrast).
It is noteworthy that in two cases with similar symptoms (progressive dyspnea and lower limb edema), a diagnosis of asthma was initially made, and only due to the persistence of the symptoms and absence of response to treatment it was possible to determine the presence of the cardiac mass. However, it is unclear whether the concomitant disease of bronchial asthma was ruled out.
In the remaining seven cases (53.95%) the diagnosis of the cardiac mass was reached while clarifying the etiology causing the symptoms.
Diagnostic imaging techniques
The most employed imaging technique for making a diagnosis was transthoracic color Doppler echocardiography (69.23%, n = 9), including the four asymptomatic cases. In the remaining five patients, this test was part of the study carried out based on the symptoms.
As previously mentioned, a thoracic CT scan allowed making a diagnosis of a patient upon hospital admission for SARS-CoV-2 pneumonia. Moreover, a thoracic CT scan with intravenous contrast allowed the identification of a mass in a patient with suspected asthmatic decompensation and the same technique was used in another patient. A thoracic CT angiography helped to make a diagnosis in another case.
Regardless of the technique used for diagnosis, the study was completed with additional techniques in 38.46% (n = 5) of the cases.
Pre-surgical and post-surgical measurements of the cardiac masses
In 61.54% (n = 8) of the patients, the imaging technique used for the diagnosis -transthoracic color Doppler echocardiography - allowed to know the size of the mass before surgery (Table 2), since a measurement was possible immediately after its identification. A different technique was needed for the other five patients: in two cases, the information provided by transthoracic echocardiography was completed with transesophageal echocardiography and, in another two, transthoracic echocardiogram after the thoracic CT was used (in one case with intravenous contrast). One patient required MRI to diagnose a myxoma (the transthoracic ultrasound showed a sessile mass with different densities with a doubtful diagnosis, while the MRI showed a hyperintense mass on T1 and T2 sequences without uptake in perfusion sequences and with heterogeneous enhancement in late enhancement sequences, suggestive of necrosis/fibrosis), so the MRI was used to measure the mass.
Table 2. Comparison of the imaging techniques employed to make the diagnosis and preoperative measurement of the cardiac masses.
| Patient | Imaging technique | |
|---|---|---|
| At diagnosis | Preoperative measurement | |
| 1 | Thoracic CT with IV contrast | TTE Doppler color |
| 2 | TTE Doppler color | ETE Doppler color |
| 3 | Thoracic CT with IV contrast | Thoracic CT with IV contrast |
| 4 | TTE Doppler color | TTE Doppler color |
| 5 | TTE Doppler color | TTE Doppler color |
| 6 | TTE Doppler color | TTE Doppler color |
| 7 | TTE Doppler color | TTE Doppler color |
| 8 | Thoracic CTA | Thoracic CTA |
| 9 | Thoracic CT | TTE Doppler color |
| 10 | TTE Doppler color | TTE Doppler color |
| 11 | TTE Doppler color | ETE Doppler color |
| 12 | TTE Doppler color | TTE Doppler color |
| 13 | TTE Doppler color | Cardiac MRI |
TEE: transesophageal echocardiogram; TTE: transthoracic echocardiogram; IV: intravenou; MRI: magnetic resonance imaging; CT: computed tomography.
All patients underwent median sternotomy, in addition to the use of extracorporeal circulation (except for the patient with the pericardial cyst). The objective of the intervention was the complete excision of the masses, which was possible in all cases except two: the patient who presented intimal sarcoma of the pulmonary artery and the one who presented a leiomyosarcoma in the left atrium. The latter died during the surgery.
Postoperative diameter was defined as the measurement made by the surgical team after the removal of the mass, before obtaining biopsies for the Department of Anatomical Pathology. Correspondence between mean pre-surgical and post-surgical diameters was very high (ICC= 0.807, 95%CI: 0.450-0.943). Mean post-surgical diameter of the masses did not differ significantly (p = 0.237) based on their location (right atrium: myxoma and thrombus, or left atrium: myxomas and leiomyosarcoma).
First checkup at hospital and first outpatient checkup
One patient died during the excision surgery. The intervention proceeded without incidents. While the surgical team was suturing, they discovered a tear in the left atrium that they were unable to resolve. The patient died due to diffuse hemorrhage.
Besides the above exitus, no other patient died and all remained asymptomatic between the period immediately after the surgery and the first in-hospital checkup. At the time of data collection, only imaging techniques aimed at controlling recurrence were considered; thus, for this study, other tests performed to monitor the postoperative period, such as chest X-rays or electrocardiograms, were not taken into consideration (Table 3). In one case, no transthoracic echocardiography was performed to show mass persistence. Excisions were successful in the rest of the patients (91.67%, n = 11). In the patient with a diagnosis of intimal sarcoma and associated thrombosis, besides an echocardiography, a CT angiography of the chest was performed (since the tumor was in the pulmonary trunk, extending to both pulmonary arteries). The results showed that the pulmonary trunk and the left pulmonary artery were free of disease, but a small filling defect persisted, which slowed the flow of the right superior lobar artery. Based on these results, the patient was offered adjuvant postoperative treatment with radiotherapy and chemotherapy.
Table 3. Data at first in-hospital checkup and first outpatient checkup.
| First checkup | ||
|---|---|---|
| In-hospital | Outpatient (after discharge) | |
| Days after surgery, mean (SD)/P50 (IQR) | 4.83 (2.17) | 34 (30-41) |
| Asymptomatic, n (%) | 12 (100) | 12 (100) |
| Imaging technique used for recurrence control, n (%) | ||
| TTE Doppler color | 11 (91.67) | - |
| Thoracic CTA | 1 (8.33) | - |
| Complementary tests used for outpatient control, n (%) | ||
| TTE Doppler color | - | 7 (58.33) |
| ECG | - | 7 (58.33) |
| PA and lateral chest X-rays | - | 9 (75.00) |
| Recurrence, n (%) | 0 | 1 (8.33) |
| Death, n (%) | 1 (7.69)* | 0 |
CT: computed tomography; CTA: computed tomography angiography; ECG: electrocardiogram; IQR: interquartile range; PA: posteroanterior view; P50: median; SD: standard deviation; TEE: transesophageal echocardiogram; TTE: transthoracic echocardiogram; -: does not apply;
*:frequency calculated over n = 13.
At the time of the first outpatient checkup after hospital discharge, all patients were asymptomatic. The frequency of transthoracic echocardiogram was lower (58.33%, n = 7) and only four patients (33.33%) underwent all three tests (echocardiogram, electrocardiogram, and chest X-ray). In ten cases (83.33%), the checkup took place one month after surgery, and in two, four months later. Partial recurrence was noted in one of the latter two cases (whose initial diagnosis had been intrapericardial cyst). Although the echocardiogram performed postoperatively in the hospital showed no alterations, the one performed during the first outpatient checkup showed a smaller lesion than the initial one. Thus, there was an intrapericardial mass of around 2 cm in the lateral region of the free wall of the right ventricle. A thoracic CT revealed that it was a heterogeneous lesion with lobulated edges and peripheral contrast enhancement, which extended towards the inferior surface of the right atrioventricular groove and the anterior mediastinum and up to the sternum. Although follow-up was recommended in two months, the patient did not return to the clinic. This was the only patient who had a recurrence.
DISCUSIÓN
In this study we exhaustively characterize symptoms in patients diagnosed with cardiac masses and cysts in our environment. The general hospital Clínica Universidad de Navarra16 serves people living in Navarre, as well as patients from other Spanish communities and from abroad. However, only 13 cases with cardiac masses and cysts have been operated in the last 20 years. Probably, a higher number of diagnoses were made at this hospital, but patients decided to undergo treatment at their places of origin or at the Department of Cardiac Surgery of Hospital Universitario de Navarra, the reference public general hospital for the area of Pamplona and the entire Chartered Community of Navarre. This fact responds to the low prevalence of cardiac tumors, less than 0.3%1. Additional data from similar studies are of great value to help improve future diagnostic performance, understand the condition of this patient population, and provide optimal clinical care.
The most frequently found type of mass in this study is myxoma (the most common benign primary tumor)8 , which is in line with other studies. One patient was diagnosed with a leiomyosarcoma, which originates from smooth muscle cells, commonly affecting abdominopelvic organs19 such as the uterus or the intestine; those that affect the heart are extremely rare and few cases have been described in the literature20. Although cardiac metastases, such as melanoma, breast, lung, or esophageal cancer17,18, are markedly more common than primary tumors5, none of the patients in this study had cardiac metastasis.
As for the location, 85% of the myxomas are reported to be found in the left atrium9, very similar to the frequency in this study (88.89%). Although sarcomas commonly affect the right side of the heart3, in this series the mass was found in the left atrium in one patient.
Cardiac tumors must be differentiated from other cardiac masses such as vegetations, thrombi, or myocardial hypertrophy3. In this series, one of the patients had a thrombus. Another patient presented an intimal sarcoma of the pulmonary trunk, an extremely unusual and aggressive cardiovascular tumor, frequently misdiagnosed for the much more common chronic pulmonary thromboembolism21. Local invasion or distant metastasis typical of sarcomas causes the patient to die within months or even weeks3.
Average age of the patients in this study was 63.08 years, consistent with the fact that cardiac masses are most common between the third and sixth decades of life10. Ten per cent of myxomas are a familial variant part of the Carney complex, an endocrine neoplasia syndrome of autosomal dominant transmission caused by a mutation in the PRKAR1A3. While sporadic forms usually appear as isolated entities in the left atrium - adhered to the interatrial septum -, the genetic variant can occur at younger ages, be multiple, and locate in the ventricles. In this series, there is no indication that the cases are of the familial variant, except for the case of the youngest patient (30 years of age); in this latter case, while performing the resection of the myxoma of the left atrium anchored in the interatrial septum, a 1-centimeter myxoma was found in the right atrium attached to the septum. No further information was available, e.g., family history of interest.
EA larger number of cardiac masses are being described with the widespread use of echocardiography as a diagnostic test22. Furthermore, the increase in the quality and use of cardiac imaging techniques in general, have also led to the rise in the diagnosis of incidental cardiac masses13. It has been estimated that the incidence of primary cardiac tumors has doubled over the past 50 years23. The increase in incidence is striking in this study; however, a causal relationship with the rise in the use of imaging techniques cannot be established due to the retrospective nature of the work and the lack of data on the global incidence of cases, as the increase in the incidence of operated cardiac masses should be accompanied by a concomitant increase in diagnoses.
The results of some studies suggest that the most prevalent cardiovascular risk factors in this patient population are smoking and dyslipidemia24, while in our series the risk factors are overweight/obesity and arterial hypertension.
Clinical manifestations are not very specific and may lead to misdiagnosis. Several cases have been described, in which the condition led to a first diagnosis of bronchial asthma25, , as occurred in this series with two cases. The typical clinical presentation for cardiac masses described in the literature is intracardiac obstruction, thromboembolism, and constitutional symptoms3,8,11,26. It is also very common for cardiac masses to mimic mitral valve disease, both stenosis (due to the prolapse of the tumor in the valve orifice) and insufficiency (due to valve trauma). The detachment of tumor fragments may lead to a thromboembolic phenomenon, and the cytokines they produce can be the cause of constitutional symptoms such as fever, asthenia, or weight loss3,8.
Patients may present symptoms, although many are asymptomatic at the time of diagnosis and the mass is found incidentally through imaging studies performed for another reason3, as in this series (30.77% asymptomatic and 46.15% incidental diagnoses).
Imaging studies should not only be aimed at making a differential diagnosis but also at evaluating the need of cardiac surgery3,8. The 2022 European Society of Cardiology (ESC) guidelines on cardio-oncology8 establish echocardiography as the preferred imaging technique for these evaluations. Although transthoracic ultrasound is often sufficient, it can be complemented with transesophageal ultrasound, which is more sensitive and specific in cases of smaller tumors or those located at unusual sites11,27,28. The study can be completed with other imaging modalities such as MRI to characterize the tissue of the mass8,29, CT or positron emission tomography (PET) to distinguish primary tumors from metastatic disease and benign tumors from malignant ones8,30. However, CT does not allow differentiating between myxomas and thrombi31, although it is the best way to detect calcifications32. In selected cases, such as young patients who present multiple myxomas, echocardiographic screening of first-degree relatives is recommended due to the possibility of a Carney complex33.
Currently, diagnosis and preoperative evaluation are usually carried out by combining different imaging techniques3,7, as in the case of 38.46% of the cases in this series.
Imaging studies allow a wide characterization, but the definitive diagnosis must be made by histological examination of the excised mass11. In a recent study that included 265 patients diagnosed with myxoma by echocardiography, only 174 (65.7%) had a pathological diagnosis of this tumor34.
Given their potential to cause serious complications, treatment of cardiac masses is imperative. Regardless of the size of the mass, the only therapeutic option is surgical excision, the result of which is usually curative3,35,36. The intervention poses very low risk or complications, embolic events being the most common11,12,37.
Patients who have undergone resection of a myxoma have a very good prognosis8,11, with a survival of 96% at 10 years38. Malignant tumors, on the other hand, have a poor prognosis. Complete surgical excision is usually impossible, so it is necessary to combine adjuvant radiotherapy or chemotherapy. In this series, two resections were incomplete: the mass located at the intimal sarcoma of the pulmonary trunk and that of the leiomyosarcoma in the left atrium.
Recurrence of cardiac masses is very low: 1-2% in sporadic myxomas (usually due to incomplete tumor resection) and 12-22% in familial cases (due to multifocal lesions initially undetected)3. Thus, follow-up with echocardiography is recommended after the intervention35,36, although it has not been established for how long. In this series, echocardiography was used in the first outpatient checkup in around half of the patients (58.33%).
There are several limitations to this study. The main one is its retrospective nature, which restricted us from collecting data such as pack years, alcohol consumption, and/or physical activity. Furthermore, its descriptive design and the lack of a comparison group limit the evaluation of the proposed causal relationships. Being a single-center study, the results have limited external validity; however, it should be noted that Clínica Universidad de Navarra serves patients from multiple national and international origins. Finally, the small number of cardiac masses distinct from myxomas should be considered. Possible improvements for future studies should consider prospective designs to avoid loss of information and the participation of multiple centers to increase external validity and detection of less frequent cardiac masses.
In conclusion, in this study, the symptoms in patients diagnosed with cardiac masses and cysts in our environment, most female, are exhaustively characterized. A case of cardiac leiomyosarcoma and a case of intimal sarcoma of the pulmonary trunk are described, two extremely rare types of tumors with limited information available in the literature. Moreover, the most frequent cardiovascular risk factors in these patients are reported. The description of these rare pathologies with such non-specific clinical presentations will help guide future diagnoses and provide optimal clinical care to these patients.
Acknowledgements
The authors would like to thank Gregorio Rábago and Estefanía Toledo for their helpful contribution to this work.
REFERENCES
1. Imazio M, Andriani M, Lobetti Bodoni L, Gaita F. Masses and tumours. En: Imazio M, Andriani M, Lobetti Bodoni L, Gaita F (eds.). Learning cardiac magnetic resonance: a case-based guide. Springer International Publishing; 2019: 145-153. doi: 10.1007/978-3-030-11608-8_10 [ Links ]
2. Cresti A, Chiavarelli M, Glauber M, Tanganelli P, Scalese M, Cesareo F, et al. Incidence rate of primary cardiac tumors: a 14-year population study. J Cardiovasc Med 2016; 17(1): 37-43. doi: 10.2459/JCM.0000000000000059 [ Links ]
3. Awtry EH. Atrial myxoma and other cardiac tumors. En: Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson JL (eds.). Harrison&s Principles of Internal Medicine. 21ª ed. Nueva York: McGraw-Hill Education, 2022. [ Links ]
4. Sultan I, Bianco V, Habertheuer A, Kilic A, Gleason TG, Aranda-Michel E, et al. Long-term outcomes of primary cardiac malignancies: multi-institutional results from the National Cancer Database. J Am Coll Cardiol 2020; 75(18): 2338-2347. doi: 10.1016/j.jacc.2020.03.041 [ Links ]
5. Tyebally S, Chen D, Bhattacharyya S, Mughrabi A, Hussain Z, Manisty C, et al. Cardiac tumors: JACC cardiooncology state-of-the-art review. JACC CardioOncol 2020; 2(2): 293-311. doi: 10.1016/j.jaccao.2020.05.009 [ Links ]
6. Poterucha TJ, Kochav J, O&Connor DS, Rosner GF. Cardiac tumors: Clinical presentation, diagnosis, and management. Curr Treat Options Oncol 2019; 20(8): 66. doi: 10.1007/s11864-019-0662-1 [ Links ]
7. Yanagawa B, Mazine A, Chan EY, Barker CM, Gritti M, Reul RM, et al. Surgery for tumors of the heart. Semin Thorac Cardiovasc Surg 2018; 30(4): 385-397. doi: 10.1053/j.semtcvs.2018.09.001 [ Links ]
8. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022; 43(41): 4229-4361. doi: 10.1093/eurheartj/ehac244 [ Links ]
9. Reynen K. Cardiac myxomas. N Engl J Med 1995; 333(24): 1610-1617. doi: 10.1056/NEJM199512143332407 [ Links ]
10. Bruce CJ. Cardiac tumours: diagnosis and management. Heart 2011; 97(2): 151. doi: 10.1136/hrt.2009.186320 [ Links ]
11. Islam AKMM. Cardiac myxomas: A narrative review. World J Cardiol 2022; 14(4): 206-219. doi: 10.4330/wjc.v14.i4.206 [ Links ]
12. Naser N, Hadziomerovic N, Bahram D, Kacila M, Pandur S. Giant right atrial myxoma with symptoms of right heart failure. Med Arch 2021; 75(1): 66. doi: 10.5455/medarh.2021.75.66-68 [ Links ]
13. Lichtenberger JP, Dulberger AR, Gonzales PE, Bueno J, Carter BW. MR imaging of cardiac masses. Top Magn Reson 2018; 27(2): 103-111. doi: 10.1097/RMR.0000000000000166 [ Links ]
14. Yang M, Yao M, Wang G, Xiao C, Wu Y, Zhang H, et al. Comparison of postoperative quality of life for patients who undergo atrial myxoma excision with robotically assisted versus conventional surgery. J Thorac Cardiovasc Surg 2015; 150(1): 152-157. doi: 10.1016/j.jtcvs.2015.01.056 [ Links ]
15. Blondeau P. Primary cardiac tumors - French studies of 533 cases. Thorac Cardiovasc Surg 1990; 38(S 2): 192-195. doi: 10.1055/s-2007-1014065 [ Links ]
16. Ministerio de Sanidad. Gobierno de España. Catálogo Nacional de Hospitales. Consultado el 19 de mayo de 2023. https://www.sanidad.gob.es/ciudadanos/prestaciones/centrosServiciosSNS/hospitales/docs/CNH_2022.pdf [ Links ]
17. Bussani R, De-Giorgio F, Abbate A, Silvestri F. Cardiac metastases. J Clin Pathol 2007; 60(1): 27. doi: 10.1136/jcp.2005.035105 [ Links ]
18. Maleszewski JJ, Bois MC, Bois JP, Young PM, Stulak JM, Klarich KW. Neoplasia and the heart: Pathological review of effects with clinical and radiological correlation. J Am Coll Cardiol 2018; 72(2): 202-227. doi: 10.1016/j.jacc.2018.05.026 [ Links ]
19. Mangla A, Yadav U. Leiomyosarcoma. Statpearls. 2022. Available in: https://www.ncbi.nlm.nih.gov/books/NBK551667. (Last Update: November 30, 2022). [ Links ]
20. Babatasi G, Massetti M, Agostini D, Galateau F, Le Page O, Saloux E, et al. Leiomyosarcoma of the heart and great vessels. Ann Cardiol Angeiol 1998; 47(7): 451-458. http://www.ncbi.nlm.nih.gov/pubmed/9772966 [ Links ]
21. Chan EY, Ravi V, Ali A, Hguyen DT, Graviss EA, MacGillivray TE, et al. Surgical management of primary pulmonary artery sarcoma. Semin Thorac Cardiovasc Surg 2023; 35(1): 53-64. doi: 10.1053/j.semtcvs.2021.10.013 [ Links ]
22. Samanidis G, Khoury M, Balanika M, Perrea DN. Current challenges in the diagnosis and treatment of cardiac myxoma. Kardiol Pol 2020; 78(4): 269-277. doi: 10.33963/KP.15254 [ Links ]
23. Oliveira GH, Al-Kindi SG, Hoimes C, Park SJ. Characteristics and survival of malignant cardiac tumors. CIRC. 2015; 132(25): 2395-2402. doi: 10.1161/Circulationaha.115.016418 [ Links ]
24. Pérez Andreu J, Parrilla G, Arribas JM, García-Villalba B, Lucas JJ, Garcia Navarro M, et al. Neurological manifestations of cardiac myxoma: Experience in a referral hospital. Neurología 2013; 28(9): 529-534. doi: 10.1016/j.nrleng.2013.10.016 [ Links ]
25. Zuwasti U, Quarrie R, Allen E, Haas C. Severe functional mitral stenosis due to a left atrial myxoma masquerading as asthma. BMJ Case Rep 2020; 13(12): e236876. doi: 10.1136/bcr-2020-236876 [ Links ]
26. Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma: A series of 112 consecutive cases. Medicine 2001; 80(3): 159-172. doi: 10.1097/00005792-200105000-00002 [ Links ]
27. Obeid AI, Marvasti M, Parker F, Rosenberg J. Comparison of transthoracic and transesophageal echocardiography in diagnosis of left atrial myxoma. Am J Cardiol 1989; 63(13): 1006-1008. doi: 10.1016/0002-9149(89)90162-8 [ Links ]
28. Engberding R, Daniel WG, Erbel R, Kasper W, Lestuzzi C, Curtius JM, et al. Diagnosis of heart tumours by transoesophageal echocardiography: a multicentre study in 154 patients. Eur Heart J 1993; 14(9): 1223-1228. doi: 10.1093/eurheartj/14.9.1223 [ Links ]
29. Beroukhim RS, Prakash A, Valsangiacomo Buechel ER, Cava JR, Dorfman AL, Festa P, et al. Characterization of cardiac tumors in children by cardiovascular magnetic resonance imaging: a multicenter experience. J Am Coll Cardiol 2011; 58(10): 1044-1054. doi: 10.1016/j.jacc.2011.05.027 [ Links ]
30. D&Angelo EC, Paolisso P, Vitale G, Foà A, Bergamaschi L, Magnani I, et al. Diagnostic accuracy of cardiac computed tomography and 18-F fluorodeoxyglucose positron emission tomography in cardiac masses. JACC Cardiovasc Imaging 2020; 13(11): 2400-2411. doi: 10.1016/j.jcmg.2020.03.021 [ Links ]
31. Scheffel H, Baumueller S, Stolzmann P, Leschka S, Plass A, Alkadhi H, et al. Atrial myxomas and thrombi: Comparison of imaging features on CT. AJR Am J Roentgenol 2009; 192(3): 639-645. doi: 10.2214/AJR.08.1694 [ Links ]
32. Shin W, Choe YH, Kim SM, Song IY, Kim SS. Detection of cardiac myxomas with non-contrast chest CT. Acta radiol 2014; 55(3): 273-278. doi: 10.1177/0284185113496561 [ Links ]
33. Carney JA, Hruska LS, Beauchamp GD, Gordon H. Dominant inheritance of the complex of myxomas, spotty pigmentation, and endocrine overactivity. Mayo Clin Proc 1986; 61(3): 165-172. doi: 10.1016/S0025-6196(12)61843-6 [ Links ]
34. Lee SH, Park JS, Park JH, Chin JY, Yoon WS, Kim HY, et al. Comparison of clinical and echocardiographic characteristics between cardiac myxomas and masses mimicking myxoma. Korean Circ J 2020; 50(9): 822. doi: 10.4070/kcj.2020.0024 [ Links ]
35. Hill M, Cherry C, Maloney M, Midyette P. Surgical resection of atrial myxomas. AORN J 2010; 92(4): 393-409. doi: 10.1016/j.aorn.2010.06.012 [ Links ]
36. Schaff HV, Mullany CJ. Surgery For Cardiac Myxomas. Semin Thorac Cardiovasc Surg 2000; 12(2): 77-88. doi: 10.1053/ct.2000.5079 [ Links ]
37. Papadopoulos K, Alexiou C, Ozden Tok O, Vannan MA. Intraoperative embolism of a right atrial myxoma: a case report. Eur Heart J Case Rep 2020; 4(6): 1-4. doi: 10.1093/ehjcr/ytaa476 [ Links ]
38. Lin Y, Xiao J, Chen J, Hong J, Peng H, Kang B, et al. Treating cardiac myxomas: a 16-year Chinese single-center study. J Cardiovasc Med 2016; 17(1): 44-53. doi: 10.2459/JCM.0000000000000114 [ Links ]
Data availability
Data will be made available on request from the corresponding author.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical statementThis study was conducted in compliance with the Declaration of Helsinki and approved by the Research Ethics Committee of the University of Navarra (project 2023.140). Patient information was coded to protect their identity.
Cite as:Gil-Korilis A, Esquíroz-Patiño C, Llamas-Llamazares A, Manrique R, Diaz-Dorronsoro A. Series of patients operated for masses and cysts in the heart in a Spanish general hospital: A 20-year experience. An Sist Sanit Navar 2023; 46(3): e1060. https://doi.org/10.23938/ASSN.1060
Received: September 11, 2023; Revised: October 16, 2023; Accepted: November 06, 2023