My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
International Microbiology
Print version ISSN 1139-6709
INT. MICROBIOL. vol.7 n.3 Sep. 2004
| RESEARCH ARTICLE | |||
|
| |||
| Fe-Si biominerals in the Vilyuchinskie hot springs, Kamchatka Peninsula, Russia
Summary. The micromorphological structure of microbial mats (biomats) from the hot springs of the Vilyuchinskaya hydrothermal system, Kamchatka Peninsula, Russia, were investigated. The Vilyuchinskie hot springs had a discharge temperature of 55–56°C and Na-Ca-HCO3-type waters rich in silicic and boric acids. Water and biomats had high concentrations of Fe, Mn, Sr, and As. Enumeration of total bacterial abundance (TBA) demonstrated a low density of bacterial populations. However, the fractions of metabolically active bacteria and respiring iron-oxidizing bacteria in the hot-spring water were high, comprising 68 and 21% of TBA, respectively. Scanning electron microscopy equipped with an energy dispersive X-ray spectrometer (SEM-EDX) showed that unicellular rod-shaped bacteria about 5-µm long predominated in the brown biomats. The mineral capsules of these bacteria contained large amounts of Fe and Si. Extracellular and intracellular particles were observed by transmission electron microscopy. Fe-oxidizing bacteria were isolated from the biomats on agar plates with selective medium. Therefore, it can be concluded that microorganisms inhabiting the biomats of the Vilyuchinskie hot springs are essential for the deposition of Fe-minerals at neutral pH. [Int Microbiol 2004; 7(3):193-198] Key words: hot springs · microbial mats · biomats · Fe-minerals · Kamchatka Peninsula | ||
| |||
| Biominerales de Fe-Si de las fuentes termales de Vilyuchiskie, en la península de Kamchatka (Rusia) Resumen. Se describe el estudio de la estructura micromorfológica de los tapetes microbianos (biomats) de las fuentes termales del sistema hidrotermal de Vilyunchinskaya, en la península de Kamchatka (Rusia). Las fuentes hidrotermales de Vilyichinskie tenían una temperatura de descarga de 55–56ºC y sus aguas son del tipo Na-Ca-HCO3, ricas en ácidos silícico y bórico. El agua y los tapetes microbianos tenían una alta concentración de Fe, Mn, Sr y As. La enumeración de la abundancia total de bacterias (ATB) mostró una baja densidad de poblaciones bacterianas. Sin embargo, en el agua de las fuentes termales las fracciones de bacterias metabólicamente activas y de bacterias respiradoras oxidadoras de Fe eran elevadas, del 68 y el 21% de ATB, respectivamente. El estudio mediante el microscopio electrónico de barrido equipado con un espectrofotómetro de rayos X dispersor de energía (SEM-EDX) mostró que en los tapetes microbianos marrones predominaban las bacterias unicelulares en forma de bacilo de unos 5 µm. Las cápsulas minerales de estas bacterias contenían gran cantidad de Fe y Si. La observación con microscopía electrónica de transmisión reveló la presencia de partículas extracelulares e intracelulares. De los tapetes microbianos se aislaron bacterias oxidadoras de Fe mediante placas de agar con medio selectivo. Por tanto, se puede concluir que los microorganismos de los tapetes microbianos de las fuentes termales de Vilyuchinskie son esenciales para el depósito de minerales de hierro a pH neutro. [Int Microbiol 2004; 7(3):193-198] Palabras clave: fuentes termales · tapetes microbianos · biomats · minerales de hierro · península de Kamchatka | Biominerais de Fe-Si das fontes termais de Vilyuchiskie, na península de Kamchatka (Rússia) Resumo. O estudo descreve a estrutura micromorfológica dos tapetes microbianos (biomats) das fontes termais do sistema hidrotermal de Vilyunchinskaya, na península de Kamchatcka (Rússia). As fontes hidrotermais tinham uma temperatura de descarga de 55–56ºC e suas águas eram do tipo Na-Ca-HCO3, ricas em ácidos sílicico e bórico. A água e os tapetes microbianos apresentavam uma alta concentração de Fe, Mn, Sr e As. A enumeração da abundância total das bactérias (ATB) mostrou uma baixa densidade das populações bacterianas. Sem dúvida, na água das fontes termais as frações das bactérias metabolicamente ativas e das respiradoras oxidadoras de Fe eram elevadas, compreendendo 68 e 21% de ATB, respectivamente. O estudo feito com microscópio eletrônico de varredura equipado com um espectrofotômetro de raios X dispersores de energía (SEM-EDX) mostrou que nos tapetes microbianos marrons predominavam bactérias unicelulares em forma de bacilo de uns 5 µm. As cápsulas minerais destas bactérias continham grande quantidade de Fe e Si. A observação com microscopia eletrônica de transmissão revelou a presença de partículas extra e intracelulares. Através do uso de placas de ágar com meio seletivo foram isoladas bactérias oxidadoras de Fe dos tapetes microbianos. Conclui-se que os microrganismos dos tapetes microbianos das fontes termais de Vilyuchinskie são essenciais para o depósito de minerais de ferro em pH neutro. [Int Microbiol 2004; 7(3):193-198] Palavras chave: fontes termais · tapetes microbianos · biomats · minerais de ferro · península de Kamchatka |
Introduction
Iron minerals are abundant in various geoecosystems. The process of Fe-mineral formation consists of three steps: Fe(II) oxidation, Fe(III) hydrolysis, and Fe(III) precipitation [20]. In natural geosystems, Fe(II) oxidation, which is the rate-limiting step, can be mediated either chemically or biologically. In acidic environments, the rate of chemical oxidation is low, and Fe-minerals are deposited due to Fe(II) oxidation by microorganisms such as Thiobacillus ferrooxidans [6,8]. The role that microorganisms play in the oxidation of ferrous iron and in the deposition of Fe-minerals at neutral pH has been enigmatic for both geochemical and microbiological reasons. Based on geochemical characteristics, it was noted that Fe(II) was chemically oxidized to Fe(III) at pH > 5 [13]. Under these conditions, microorganisms could be involved in the process of Fe-mineral deposition and thus compete with the chemical oxidation occurring in hydrothermal systems when the iron concentration is high and the content of dissolved oxygen is low in the surrounding water. The latter occurs when water is discharged from wells or deep-water vents, leading to the formation of anoxic-oxic transition zones consisting of regions of low-oxygen concentration. There are several examples of the presence and direct impact of microorganisms on the formation of Fe-minerals [2,3,5,9,12]. At the Loihi Seamount hydrothermal vents, in the Hawaiian archipelago, anaerobic neutrophilic Fe-oxidizing bacteria were abundant in hydrous ferric oxides (HFOs), and most cells appeared to be tightly associated with the Fe(hydro)oxides [9]. An effect of bacteria on the precipitation of HFOs and a role for the bacterial cell wall in iron deposition have been reported in Fe-stalactite formation [12]. The estimated rate of iron precipitation in the field was in good agreement with bulk estimates of Fe-stalactite growth rates and was about four orders of magnitude faster than the expected inorganic precipitation rate [12]. It was suggested that iron, which is not used as an energy source for Fe-bacteria, might be an essential structural component of the sheaths [4,12]. In addition, the importance of microorganisms in the formation of modern hydrothermal iron-silica deposits has been reported [7,11]. Based on observations of microbial fossil assemblages, Ferris et al. [10] suggested that microfossils formed as a result of mineral precipitation and would probably be best preserved if they had been previously embedded in a fibrous silica matrix. Whereas thermal-spring microbes are apparently poorly preserved in iron(hydro)oxides or carbonates, fossilization by silica can provide enduring evidence of life, and preservation of microbial fossils by silicification has been documented in ancient thermal springs [1,7,14,17,23].
The Kamchatka Peninsula, located between the Sea of Okhotsk and the Bering Sea, is the only example of modern volcanic activity in Russia. Over 150 groups of hot springs occur in four geothermal provinces. Southern Kamchatskaya geothermal province is south of Petropavlovsk-Kamchatskii, which is the capital of the Kamchatka Region. The Mutnovskoe geothermal region, located there, consists of the Mutnovskoe, Zhirovskoe, and Vilyuchinskoe hydrothermal fields [22]. These hydrothermal systems were initially studied in the 1960s and 1970s. Vakin, Sugrobov and Kiryukhin were the first to investigate the Mutnovskoe geothermal field, but their interests were restricted to chemical and geological studies of the water and of modern deposits [22]. Later, Okrugin, in 1994 and 1995 [Okrugin VM (1995) WRI-8 post-session field trip to Kamchatka. Part I: Mutnovsky geothermal field. 8th International Symposium on Water-Rock Interaction 1-29], and Chudaev et al., in 2000, reported high concentrations of iron in the water of the Dachnye and Vilyuchinskie hot springs [15, 22]. Moreover, Okrugin et al. [Biogenic mineral formation in modern hydrothermal systems of Kamchatka Peninsula (Mutnovsko-Asachinskii volcanogenic-ore forming center). Abstract of the 1st International Symposium "Life and Rock" (2002), pp. 100-101], Tazaki et al. [22], and Saji et al. [19] noted the presence of Fe-containing minerals in modern hydrothermal deposits from the springs. Accordingly, the diversity, structure, and function of the microbial communities inhabiting the hot-spring water and biomats are of great interest to geomicrobiologists.
In previous investigations, we studied four hot springs, located at the Vilyuchinskoe hydrothermal field, which showed similar chemical characteristics [22]. At the Vilyuchinskie hot springs, water discharges at 55-56ºC and flows downstream from the travertine, which consists of calcite, aragonite, opal, oxides and hydroxides of Fe and Mn, sulfides of Fe, Cu, Zn, Hg, and native Au [Okrugin VM, Belkova NL, Tazaki K (2002) Biogenic mineral formation in modern hydrothermal systems of Kamchatka Peninsula (Mutnovsko-Asachinskii volcanogenic-ore forming center). Abstract of the 1st Int. Symp. "Life and Rock"), pp. 100-101]. Deposits are 1 to 3-m thick, thinly laminated, have a sponge-like structure, and extend for more than 1 km along the drainage channels. While only low densities of bacterial populations were observed in the hot-spring waters, the fractions of metabolically active bacteria were high, ranging from 27% to 91% [22]. We suggested that microorganisms contribute to the formation of minerals at these springs. Thus, the main objective of the present study was to investigate hot-spring water and biomats from the Vilyuchinskaya hydrothermal system in order to detect microorganisms actively involved in Fe-minerals formation and deposition.
Materials and methods
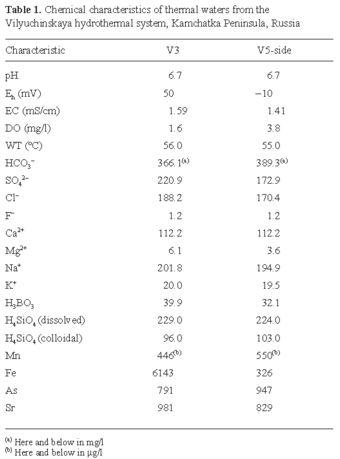
Sampling and field measurement of water quality. Samples of thermal water and biomats were collected in October, 2002 and September, 2003 from two hot springs of the Vilyuchinskaya hydrothermal system (Fig. 1A). These springs were: (1) a drilled borehole and (2) a natural source located on a travertine dome. Tazaki et al. [22] named these sites V3 and V5-side (Fig. 1B, C). Brown biomats were collected from the drilled borehole (V3) and green biomats were collected from the travertine dome (V5-side). The biomats were fixed with glutaraldehyde (2.5% final concentration) immediately after sampling and stored at 4ºC until further use. Physicochemical variables of the hot-spring water (pH, oxidation-reduction potential [Eh], electrical conductivity [EC], dissolved oxygen [DO], and water temperature [WT]) were measured in the field. Eh was measured using an Eh-meter (Horiba) and recalculated as electrode potential vs. the standard hydrogen electrode (Table 1).
Culture of the bacteria. The growth medium for iron-oxidizing bacteria (Fe-medium) consisted of (per liter): 0.5 g (NH4)2SO4, 0.5 g NaNO3, 0.5 g K2HPO4, 0.5 g MgSO4, 5.9 g FeSO4, 1 g tryptone. For solid medium (Fe-agar), 15-20 g agar (Difco) were added. The pH of the medium was adjusted to 6.8-7.0. After incubation at 20°C for 2 to 6 weeks, bacteria were subcultured and purified on Fe-agar plates. For microscopy, iron-oxidizing bacteria were cultivated in liquid Fe-medium for 2 days. The cells were subsequently fixed with 1% glutaraldehyde and visualized by transmission electron microscopy (TEM, JEOL 2000EX).
Fluorometric staining of bacteria. To enumerate the total number of bacteria, aliquots of unfiltered hot-spring water were fixed immediately after collection with 1% glutaraldehyde. Preserved samples were stored at 10°C and analyzed within 10-14 days after sampling. The total number of bacteria for each water sample was determined by direct cell enumeration using 4´,6-diamidino-2-phenylindol (DAPI). Cells that had either hydrolyzed the diacetate groups in 5-carboxyfluorescein diacetate (CFDA) into fluorescent carboxyfluorescein (CFDA+ cells) or reduced the tetrazolium salt 5-cyano-2,3-ditolyltetrazolium chloride (CTC) to its fluorescent formazan compound (CTC+ cells) were defined as metabolically active. CFDA was added to a final concentration of 0.01 mM [16]. Samples were incubated statically for 10 min in the dark. The CTC assay was done using a procedure modified from Rodriguez et al. [18]. Samples were diluted 1:2 with liquid medium for Fe-oxidizing bacteria, and 5 mM CTC (final concentration) was added to each sample followed by a 20-h incubation in the dark. The negative control comprised samples fixed with 1% (final concentration) glutaraldehyde. Each water sample was filtered through a 0.22-µm black membrane filter (Millipore) with a backing filter. The filter was wet-mounted on a microscope slide using non-fluorescent immersion oil and stored at 4°C in the dark for 20 min before counting.
Counting of the bacteria. Total bacterial abundance as well as the presence of enzymatically active bacterial cells and respiring iron-oxidizing bacteria were determined for the brown biomats obtained from the Vilyuchinskie V3 hot springs. Stained cells were counted at a magnification of 100× (plane objective) using an epifluorescence microscope (Nikon EFD-3) outfitted with an ocular grid and appropriate optical filter sets. For each sample, more than 500 bacteria-like particles were enumerated. The mean abundances and standard deviations (SD) were calculated by counting 10 to 20 fields chosen randomly for each filter. The percentage of active bacterial cells was calculated as the number of CFDA+ or CTC+ cells divided by the total bacterial abundance (as detected by DAPI staining) ×100.
Chemical analyses of hot-spring water and biomats. The content of the most abundant ions, H3BO3, and H4SiO4 (dissolved and colloidal), in hot-spring water was studied by colorimetry and flame photometric spectrometry (model SP-2900) at the Central Chemical Laboratory of the Institute of Volcanology Far-Eastern Division of the Russian Academy of Sciences (FED RAS), Petropavlovsk-Kamchatskii. The elemental composition of water samples was determined using inductively coupled plasma-mass spectroscopy and inductively coupled plasma-atomic emission spectrometry (ICP-MS and ICP-AES) in the Analytical Laboratory of the Institute of Problem Microelectronic Technology of Russian Academy of Sciences (RAS), Chernogolovka. The chemical composition of biomats was analyzed by photocolorimetry (e.g. SiO2, Al2O3, Fe2O3, TiO2, and P2O5), atomic absorption (e.g. MgO, CaO, and MnO), and flame photometry (e.g. Na2O, K2O). FeO was analyzed volumetrically. The mass loss of the sample after drying at 110°C was defined as the mass of adsorbed water, H2O(-). Subsequent ignition at 1000-1100°C in Penfield tubes revealed structural OH, H2O(+).
Scanning and transmission electron microscopy. Samples were freeze-dryied for scanning electron microscopy (SEM) [21]. One drop of the biomat was mounted onto a filter with a pore diameter 0.45 µm (JEOL). The sample was washed, fixed with t-butyl alcohol, frozen in liquid nitrogen, and dried under low vacuum. The samples were observed with a scanning electron microscope (JEOL-JSM-5200LV) equipped with an energy-dispersive X-ray spectrometer (Philips-EDAX PV9800 STD). Additionally, the fixed sample was mounted on a microgrid and observed with a transmission electron microscope (JEOL 2000EX, operated at an accelerating voltage of 80 to 120 kV) at different magnifications. During TEM, electron diffraction analysis was also carried out in order to identify the mineralogical structure of the particles.
Results
Chemical characteristics of thermal water and biomats from the Vilyuchinskaya hydrothermal system. Tables 1 and 2 list the chemical composition of the thermal water and biomats. Water at both measuring points had a neutral pH (6.7). The Eh values reflected the oxidative effect of the thermal water of the V3 spring (50 mV) and the slightly reductive effect of the V5-side spring (-10 mV). The DO concentrations were low at both sites. A general feature of the ion composition was the predominance of HCO3- compared to SO42-, and of Na+ and Ca+ compared to other cations. The sodium concentration was about twice as high as the calcium concentration. Thus, the Vilyuchinskie thermal waters are of the Na-Ca-HCO3-type. Additionally, a high concentration of dissolved and colloidal H4SiO4 was detected in both hot springs. Of the 72 elements analyzed, 47 were at concentrations under the detection limits. Table 1 lists the concentrations of selected elements. Among the heavy metals, Mn, Fe, As, and Sr were present in high concentrations. Note that the content of Fe in the water from the V3 hot springs (6143 µg/l) was about 20 times higher than that in the water of the V5-side hot springs (326 µg/l). The concentration of the other elements in the waters from the two hot springs did not vary remarkably. High levels of As were detected at both sampling sites. Table 2 lists the results of chemical analyses of the brown (V3) and green (V5-side) biomats.
Enumeration of bacteria in the hot-spring water. The total bacteria count of DAPI-stained cells was 9.40 × 103 cells/ml, indicating a low bacterial density. However, enumeration of CFDA-stained cells revealed a high percentage of active bacteria in the hot-spring water [68% (6.40 × 103 cells/ml)]. Respiratory activity, as determined by the reduction of CTC, was measured in Fe-oxidizing bacteria grown in selective medium under optimal conditions. The fraction of CTC+ bacteria was 21%. Additionally, inoculants from the Vilyuchinskie hot-spring biomats were cultured on Fe-agar plates and growth of iron-oxidizing bacteria was obtained after a 2-week incubation.
Micromorphology of the brown and green biomats. SEM revealed the highly abundant presence of curved rods, about 5-µm long, in the brown biomats (Fig. 2A). By contrast, in the green biomats there was a high diversity of microorganisms, comprising bacteria of very diverse shapes and sizes, present as single cells or in colonies, and aggregated with mineral particles (Fig. 2B). SEM-EDX analysis of bacterial cells from the brown biomats showed strong Fe and Si peaks accompanied by peaks for Al and Ca (Fig. 2C), whereas in green biomats the peaks for Si and Fe were accompanied by a characteristic P peak (Fig. 2D).
TEM revealed mineral-encrusted capsules around bacterial cells and highly electron-dense amorphous particles located inside the cells (Fig. 3A, B, C). Commonly, the capsules of bacteria from brown biomats consisted of amorphous particles with a diameter of about 50 nm (Fig. 3A). Rarely, particles of 200 nm were observed either attached to the bacterial capsules or inside them (Fig. 3B). Fig. 3C shows intracellular mineral particles with a diameter of about 100 nm. Particles attached to the cell walls of iron-oxidizing bacteria grown on selective medium had an amorphous structure (Fig. 3D).
Discussion
The waters of the two hot springs were very similar with respect to the temperature of the discharging water, the pH, and the ion and chemical compositions. However, a comparison of the oxidation-reduction potential showed the oxidative effect of the thermal water of the Vilyuchinskie V3 springs. Additionally, a high iron concentration was measured in the hot-spring water from this site (Table 1), and ferric iron predominated over the ferrous form at a ratio of 52.7 in the brown biomats (Table 2). Thus, at the V3 site Fe(II) oxidation is extremely enhanced. The amorphous mineral particles observed around the bacterial cells from cultured iron-oxidizing bacteria suggested that Fe(II) oxidation should be catalyzed or mediated by these microorganisms. Kasama and Murakami [12] reported that microorganisms increase the rate of iron oxidation by about four orders of magnitude over the expected inorganic precipitation rate. By contrast, in the green biomats (V5-side), the ferric-to-ferrous iron ratio was less than 1, indicating the existence of stable complexes of Fe(II). The rate of iron oxidation was much lower than that in the brown biomats. It would be interesting to identify the reason for the great difference between the biomats from these two hot springs, which are located in the same area. In V3, iron bacteria catalyze biogenic Fe(II) oxidation and increase the rate of Fe-mineral deposition. Moreover, the waters of the Vilyuchinskie springs are rich in silicic acid and there is a rapid rate of silicification during water discharge and cooling. These processes take place simultaneously. As a consequence, Fe-Si biominerals precipitate around bacterial cells and form the mineral-encrusted capsules revealed by TEM (Fig. 3).
Geochemical monitoring of waters at the Vilyuchinskaya hydrothermal system has been carried out over the last decade [Okrugin VM, Belkova NL, Tazaki K (2002) Biogenic mineral formation in modern hydrothermal systems of Kamchatka Peninsula (Mutnovsko-Asachinskii volcanogenic-ore forming center). Abstract of the 1st Int. Symp. "Life and Rock"), pp. 100-101], [15,19,22]. Our findings agree with previous data and with the intra-seasonal variations in mineral formation that have been reported. Therefore, we can conclude that Fe-Si-mineral deposition occurs in thermal waters saturated with iron and silicic acid, and that have a temperature of approximately 56ºC and a neutral pH. Iron-oxidizing bacteria are abundant in hot-spring biomats, whose thermal waters are rich in iron. The ability of iron bacteria in the biomats to deposit Fe-minerals is due to the specific properties of their cell walls and/or their metabolic pathways. The accelerated processes of iron oxidation, silicification, and deposition of Fe-Si-minerals that occur in the brown biomats of the Vilyuchinskaya hydrothermal system can be attributed to the impact of rod-shaped bacteria inhabiting the hot-spring waters and biomats.
Acknowledgements. The authors thank Svetlana Sergeeva, Valentina Dunin-Barkovskaya, and Anna Okrugina of the Central Chemical Laboratory of the Institute of Volcanology FED RAS, and Vasilii Karandashev of the Analytical Laboratory of the Institute of Problem Microelectronic Technology RAS, for their assistance with chemical analyses. This work was supported by a grant from the Japanese Ministry of Education, Science and Culture, Science and Technology to Kazue Tazaki. N. L. Belkova thanks the Japanese Government and the Ministry of Education, Science and Culture for providing scholarship for the study.
References
1. Allen CC, Albert FG, Chafetz HS, Combie J, Graham CR, Kieft TL, Kivett SJ, McKay DS, Steele A, Taunton AE, Taylor MR, Thomas-Keprta KL, Westall F (2000) Microscopic physical biomarkers in carbonate hot springs: implications in the search for life on Mars. Icarus 147:49-67 [ Links ]
2. Brown DA, Sawicki JA, Sherriff BL (1998) Alteration of microbially precipitated iron oxides and hydroxides. Am Mineral 83:1419-1425 [ Links ]
3. Brown DA, Sherriff BL, Sawicki JA, Sparling R (1999) Precipitation of iron minerals by a natural microbial consortium. Geochim Cosmochim Acta 63:2163-2169 [ Links ]
4. Carlile MJ, Dudeney AWL (2000) A microbial mat composed of iron bacteria. Microbiology 146:2092-2093 [ Links ]
5. Chaudhuri SK, Lack JG, Coates JD (2001) Biogenic magnetite formation through anaerobic biooxidation of Fe(II). Appl Environ Microbiol 67:2844-2848 [ Links ]
6. Das T, Chaudhury GR, Ayyapan S (1998) Use of Thiobacillus ferroxidans for iron oxidation and precipitation. BioMetals 11:125-129 [ Links ]
7. Duhig NC, Davidson GJ, Stolz J (1992) Microbial involvement in the formation of Cambrian sea-floor silica-oxide deposits, Australia. Geology 20: 511-514 [ Links ]
8. Ehrlich HL (1995) Geomicrobiology, 3rd edition. Marcel Dekker Inc., New York [ Links ]
9. Emerson D, Moyer CL (2002) Neutrophilic Fe-oxidizing bacteria are abundant at the Loihi Seamount hydrothermal vents and play a major role in Fe oxide deposition. Appl Environ Microbiol 68:3085-3093 [ Links ]
10. Ferris FG, Beveridge TJ, Fyfe WS (1986) Iron-silica crystallite nucleation by bacteria in a geothermal sediment. Nature 320:609-611 [ Links ]
11. Hamade T, Konhauser KO, Raiswell R, Goldsmith S, Morris RC (2003) Using Ge/Si ratios to decouple iron and silica fluxes in Precambrian banded iron formation. Geology 31:35-38 [ Links ]
12. Kasama T, Murakami T (2001) The effect of microorganisms on Fe precipitation rates at neutral pH. Chem Geol 180:117-128 [ Links ]
13. Langmuir D (1997) Aqueous environmental geochemistry. Prentice-Hall, Englewood Cliffs, New Jersey [ Links ]
14. Oehler JN. (1976) Experimental studies in Precambrian paleontology: structural and chemical changes in blue-green algae during simulated fossilization in synthetic chert. Geol Soc Am Bull 87:117-129 [ Links ]
15. Okrugin VM, Stefanov JM, Shuvalov RA, Okrugina AM, Stepanov II (1994) On ecological-geochemical monitoring in Kamchatka (the Mutnovsky-Asachanisky ore region). Mineral Magazine 58A:671 [ Links ]
16. Porter J, Diaper J, Edwards C, Pickup R (1995) Direct measurements of natural planktonic bacterial community viability by flow cytometry. Appl Environ Microbiol 61:2783-2786 [ Links ]
17. Renaut RW, Jones B, Tiercelin J-J (1998) Rapid in situ silicification of microbes at Loburu hot springs, Lake Bogoria, Kenya Rift Valley. Sedimentology 45:1083-1103 [ Links ]
18. Rodriguez GG, Phipps D, Ishiguro K, Ridgway HF (1992) Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl Environ Microbiol 58:1801-1808 [ Links ]
19. Saji I, Nishikawa O, Belkova N, Okrugin V, Tazaki K. (2004) Chemical and microbiological investigations of hot spring deposits found at the hydrothermal systems of Kamchatka Peninsula, Russia. The Science Reports of Kanazawa University 48:73-106 [ Links ]
20. Singer PC, Stumm W (1970) Acidic mine drainage: the rate-determining step. Science 167:1121-1123 [ Links ]
21. Suzuki T, Shibata M, Tanaka K, Tsuchida K, Toda T (1995) A new drying method: low vacuum SEM freeze drying and its application to plankton observation. Bull Plankt Soc Japan 42:53-62 [ Links ]
22. Tazaki K, Okrugin V, Okuno M, Belkova N, Islam ABM R, Chaerun SK, Wakimoto R, Sato K, Moriichi S (2003) Heavy metallic concentration in microbial mats found at hydrothermal systems, Kamchatka, Russia. The Science Report of Kanazawa University 47:1-48 [ Links ]
23. Walter MR, Bauld J, Brock TD (1972) Siliceous algal and bacterial stromatolites in hot springs and geyser effluents of Yellowstone National Park. Science 178:402-405 [ Links ]