My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
International Microbiology
Print version ISSN 1139-6709
INT. MICROBIOL. vol.7 n.4 Dec. 2004
| REVIEW ARTICLE | |||
|
| |||
| Organic matter in meteorites
Summary. Some primitive meteorites are carbon-rich objects containing a variety of organic molecules that constitute a valuable record of organic chemical evolution in the universe prior to the appearance of microorganisms. Families of compounds include hydrocarbons, alcohols, aldehydes, ketones, carboxylic acids, amino acids, amines, amides, heterocycles, phosphonic acids, sulfonic acids, sugar-related compounds and poorly defined high-molecular weight macromolecules. A variety of environments are required in order to explain this organic inventory, including interstellar processes, gas-grain reactions operating in the solar nebula, and hydrothermal alteration of parent bodies. Most likely, substantial amounts of such organic materials were delivered to the Earth via a late accretion, thereby providing organic compounds important for the emergence of life itself, or that served as a feedstock for further chemical evolution. This review discusses the organic content of primitive meteorites and their relevance to the build up of biomolecules. [Int Microbiol 2004; 7(4):239-248] Key words: primitive meteorites · prebiotic chemistry · chemical evolution · origin of life | ||
| |||
| Materia orgánica en meteoritos Resumen. Algunos meteoritos primitivos son objetos ricos en carbono con una gran variedad de moléculas orgánicas, lo que hace de ellos un registro muy valioso de la evolución química orgánica en el universo antes de la aparición de los microorganismos. Las familias de compuestos comprenden, entre otros, hidrocarburos, alcoholes, aldehídos, cetonas, ácidos carboxílicos, aminoácidos, aminas, amidas, heterociclos, ácidos fosfónicos, ácidos sulfónicos, compuestos relacionados con azúcares y macromoléculas poco definidas de alto peso molecular. Para explicar el origen de este material orgánico diverso, se requieren varios ambientes, tales como procesos interestelares, reacciones gas-sólido en la nebulosa solar y la alteración hidrotermal de los cuerpos asteroidales originales. Es muy probable que una gran cantidad de ese material orgánico llegara a la Tierra durante los últimos estadios de su formación y que su presencia resultara crucial para el origen de la vida o que sirviesen como material nutritivo para una evolución química posterior. Esta revisión se centra en el contenido orgánico de los meteoritos más primitivos y en su papel en la síntesis de biomoléculas. [Int Microbiol 2004; 7(4):239-248] Palabras clave: meteoritos primitivos · química prebiótica · evolución química · origen de la vida | Matéria orgânica nos meterioritos Resumo. Alguns meterioritos primitivos são objetos ricos em carbono com uma grande variedade de moléculas orgânicas, o que os fazem um registro muito valioso da evolução química orgânica no universo antes da aparição dos microrganismos. As famílias dos compostos incluem hidrocarbonados, álcoois, aldeídos, cetonas, ácidos carboxílicos, aminoácidos, aminas, amidas, heterociclos, ácidos fosfônicos, ácidos sulfônicos, compostos relacionados com açúcares e macromoléculas pouco definidas de alto peso molecular. Para explicar a origem deste material orgânico, se requer vários ambientes, tais como processos interestelares, reações gás-sólido na nebulosa solar e a alteração hidrotermal dos corpos asteroidais originais. É muito provável que uma grande quantidade deste material orgânico tenha chegado à Terra durante os últimos estágios de sua formação e que sua presença tenha sido crucial para a origem da vida ou como material nutritivo para uma evolução química posterior. Esta revisão é centrada no conteúdo orgânico dos meterioritos mais primitivos e em seu papel na síntese de biomoléculas. [Int Microbiol 2004; 7(4):239-248] Palavras chave: meteoritos primitivos · química prebiótica · evolução química · origem da vida |
This article is dedicated to Prof. John Oró (October 26, 1923-September 2, 2004).
Introduction
Like a carpentry shop littered with wood shavings after the work is done, debris left over from the formation of the Sun and planets is scattered throughout the inner solar system in the form of asteroids. Since the beginning, the Earth, the Moon and all of the planets have been hit by fragments of these objects from space. In addition to pieces of asteroids, Moon and Martian rocks have been found among the thousands of meteorites collected on Earth. In the past, meteorite falls were poorly understood phenomena and were frequently attributed to divine intervention. Many exaggerated stories developed and meteorites were often considered to be religious objects, some of which have been preserved in churches, temples, and burial chambers around the world. Nowadays, there are expeditions whose goal is to collect meteorites in hot and cold deserts, including the Sahara and Antarctica, and space missions to planetary bodies aimed at providing new opportunities for scientific advancement. One of the most important findings regarding such bodies is that comets and certain types of meteorites contain organic molecules formed in space that may have had a relevant role in the origin of the first microorganisms on Earth. This review deals with the organic inventory of meteorites. Another review (to be published in the next issue of Int Microbiol) will focus on the organic content of comets.
Space debris and the primitive soup
It is widely accepted that, for life to have appeared on Earth, a primordial soup of simple organic molecules was first needed, as suggested independently by Russian biochemist Oparin, in 1924 [38], and British biologist Haldane, in 1929 [21]. The origin of the primordial soup was identified, in 1953, with the famous Miller experiment [35,36]. When a gaseous mixture of hydrogen, water, methane and ammonia was subjected to electrical discharges simulating the effect of atmospheric storms in what was thought to be the early atmosphere of the Earth, an organic "soup" was produced that contained a wide variety of organic molecules, including amino acids, from which proteins are built. At sufficiently high energy, this reducing atmosphere generated hydrogen cyanide and aldehydes, which through the Strecker reaction resulted in the subsequent production of amino acids.
Nowadays, however, the model of a reducing primitive atmosphere has been abandoned for various reasons. For example, if the Earth-Moon system formed when a giant-sized body hit the Earth [37], the impact would have blown the entire atmosphere of the Earth out into space. Therefore, an oxidizing atmosphere rich in carbon dioxide, nitrogen and water, and derived from volcanic processes, has instead been assumed to have been present when life originated on Earth [23,24]. This model appears to be far more convincing. In fact, the oxidizing atmosphere of early Earth would have resembled that of its two neighboring planets, Venus and Mars, and not that of far more massive and distant giant planets, such as Jupiter and Saturn, as in the Miller experiment. However, if the Miller experiment is performed using an oxidizing atmosphere, organic molecules are no longer obtained with satisfactory yields. So, how and where did the building blocks of the primordial soup originate?
As will be discussed below, comets and primitive meteorites are filled with water and organic molecules. Consequently, and taking into account the fact that, since their formation, comets and meteorites have collided with our planet time after time, in 1961 Oró proposed explicitly that water and the precursors of organic matter on Earth came from the comets that bombarded it [39]. This idea was later elaborated on by Delsemme [14], Anders [1], and Chyba and Sagan [10]. In 1908, Chamberlin had already suggested a similar role for organic matter from asteroidal bodies [9]. The idea that a significant fraction of the carbon compounds required for life had an extraterrestrial source may be startling, but we should keep in mind that we, each of us, have part of a star inside us. The iron in hemoglobin that allows us to breath, and the calcium in bones, for example, were made in stars and distributed by means of nuclear explosions that ended their life even before our solar system was born (Fig. 1).
Carbonaceous chondrites. Murchison, a keystone
It was a Sunday morning, September 28, 1969, in the town of Murchison, Australia. During the time that most residents were attending church, a bright, dramatic fireball appeared, signalling the arrival of hundreds of meteorites. The specimens were scattered across an 8-km2 area within the town, and people gathered them easily from their yards, recovering more than 500 kg of the meteorite material. The Murchison meteorite belonged to the CM carbonaceous chondrite family. Most carbonaceous chondrites are primitive stony meteorites older than 4.5 Gyr that contain up to 10% of their weight in water and more than 3% of their weight in carbon [13]. They are classified by subtle chemical compositional differences on the basis of their similarity to mainly seven, prototypical specimens (Table 1, [34]).
The CI and CM carbonaceous chondrites are the richest in organic matter and water, and for that reason they are very friable and fragile. They must be collected quickly after their fall, before the weather has a chance to destroy them. This is one of the reasons why Murchison has been a crucial stone; hundreds of meteorites were recovered just after they fell, and there was almost no weathering or contamination (Fig. 2). Another unique circumstance coincided with Murchison's fall in 1969. At that time, laboratories all around the world were set up for the study of the rocks that the Apollo program had brought from the Moon. Murchison was the right meteorite that fell at the right place and at the right time. Most of the work done on meteorite organic chemistry has been carried out on Murchison. Other carbonaceous chondrites whose organic matter has also been studied are Cold Bokkeveld (South Africa, 1838), Orgueil (France, 1864), Murray (USA, 1950) and Allende (Mexico, 1969).
On the morning of January 18, 2000, an asteroid weighing approximately 200 to 250 tons and five to seven meters across impacted the Earth's atmosphere. The result was that hundreds of meteorites fell in a remote, cold arctic area between British Columbia and Yukon territories, Canada, in a strewn over a field 16 km long and 3 km wide. A week later, the first meteorite fragments were found by a nearby resident. The meteorite turned out to be a rare, primitive CI/CM chondrite very rich in organic compounds; it contained 3.6% carbon by weight [6]. A field effort consisting of 234 people, a unique effort in the history of meteoritics, recovered hundreds of specimens that had remained frozen in ice and never touched by human hands. This carbonaceous chondrite, named Tagish Lake after the frozen lake where it fell, has opened a new door on the search of organic compounds in meteorites. The fact that the fragments had been kept in a continuously frozen state minimized the loss of organic molecules and potential contamination.
Carbonaceous chondrites are very special meteorites. They are considered to be among the most primitive solar system materials on the basis of their chemical composition. With the exception of very volatile elements, including hydrogen, helium, carbon, nitrogen and oxygen, carbonaceous chondrites have an elemental composition that corresponds to that of the Sun. Taking into account that the solar system's elemental composition is equivalent to the chemistry of the Sun, since the latter contains more than 99% of the mass of the whole system, the close correspondence between the elemental composition of the Sun and carbonaceous chondrites indicates that this type of meteorites has not experienced processes leading to extensive chemical fractionation, like those that have altered all the rocks on Earth, for example. For that reason, carbonaceous chondrites provide precious insight allowing us to look back into the past.
Making a chondrite
Carbonaceous chondrites represent less than 5 % of all recovered meteorites and belong to the general group of stony meteorites called chondrites. The name ‘chondrite' derives from the ancient Greek word chondros, meaning ‘seed'. The chondros in these meteorites are millimeter-sized, globular bodies, called ‘chondrules', and are mainly composed of simple silicates, such as olivine and pyroxene. Chondrules are believed to have formed by instantaneous, brief, high-temperature events in the solar nebula that melted nebular dust to form molten droplets, which were quenched by subsequent rapid cooling [34]. As an example, the cover photograph of this issue shows a polished cross-section of the NWA1465 carbonaceous chondrite, displaying numerous chondrules.
Another outstanding component of chondrites are the irregularly shaped white inclusions. Due to their distinctive chemical composition, they are commonly called calcium-aluminum inclusions, or CAIs, and are especially abundant in CV carbonaceous chondrites [27]. Most CAIs have concentric structures formed by layers of different minerals that crystallized at high temperatures. For this reason, they are thought to have been among the first materials in chondrites to form [20]. Before 1969, CV carbonaceous chondrites were available only in gram-sized amounts, but shortly after midnight on February 8, 1969, several tons of this type of meteorite precipitated around Pueblito de Allende, Mexico. They fell over a field exceeding 280 km2, the largest strewn field ever known, and specimens are still being found after thirty years of searching. Allende is the largest carbonaceous chondrite ever have fallen.
Some chondrites are sprinkled with small metal chunks. These consist of intergrown iron-nickel alloys with different compositions and crystal structures. The amount of accreted metal is used to classify chondrites into different families. Enstatite chondrites (named for their high abundance of the mineral enstatite, MgSiO3) contain much more metal than other chondrite types; carbonaceous chondrites contain the least, and ordinary chondrites, the most abundant type, are intermediate. The whole assemblage in chondrites consist of undifferentiated conglomerates of low- and high-temperature minerals of incompatible compositions that preserve their unique imprints. For that reason, they are referred to as unequilibrated meteorites [34]. Chondrules, CAIs, metal, and other less abundant components in chondrites are cemented by an extremely fine-grained matrix. Usually, the matrix materials comprise tiny silicate grains of olivine and pyroxene, along with sulfides, oxides, feldspathoids, and clay minerals [7]. It is within the matrix that organic compounds are encountered in carbonaceous chondrites and, more specifically, in matrices containing abundant clays [41].
Sampling asteroids
Although thousands of meteorites have been recovered, only in a few cases is their point of departure exactly known. In 1959, when an ordinary chondrite impacted at Pribram, near Prague, Czech Republic, several cameras photographed it by chance. It was the first time that an orbit prior to Earth capture could be determined for a recovered meteorite [8]. Since then, six more meteorites have been recovered and their orbits precisely determined from photographic records: Lost City, in Oklahoma (1970); Innisfree, in Alberta (1977); Peekskill, in New York (1992); and, more recently, Moravka, in Czech Republic (2000); Tagish Lake, in Canada (2000); and Neuschwanstein, in Austria (2002). All of these meteorites are ordinary chondrites, except Tagish Lake, which is a carbonaceous chondrite that was also observed by satellites in Earth orbit. Their most interesting feature is that the aphelion of the orbits is, in all cases, located within the asteroid belt between Mars and Jupiter, which demonstrates that chondrites derive from asteroids.
Asteroids were never assembled into one planet that later on was disrupted. It is believed that, in the early stages of planetary formation, the large gravitational field of the giant Jupiter prevented the formation of any large planetary body within its sphere of influence [34]. For that reason, in its vicinity asteroids could never have accreted into a planet. Owing to this particular circumstance, today there are small pieces of rocky cosmic debris available as they formed 4.5 billion years ago. The total mass of all asteroids taken together is less than that of the Moon.
The surfaces of asteroids
Asteroids generate no light of their own, they merely reflect sunlight. By studying the properties of sunlight reflected from the surfaces of asteroids, we are able to infer their composition. These reflectance spectra can then be compared to those obtained from the various types of powdered meteorites measured in the laboratory, providing an interesting basis for asteroid classification [17]. Type-C and -D asteroids appear to be possible sources of carbonaceous chondrites. The largest known asteroid is Ceres, which is of the type-C, and water-bearing clays and possibly organic material have been recognized on its surface that are reminiscent of the mineralogy of the matrix of CI and CM carbonaceous chondrites [7]. Type-S asteroids have absorption bands indicating the presence of olivine, pyroxene, and iron-nickel metal. They are good potential candidates from which ordinary chondrites might be derived. Type-E and -R asteroids are associated with enstatite chondrites. These asteroidal classes are not distributed homogeneously within the asteroid belt. Most type-E and -R asteroids are located at its inner part, whereas type-C and -D asteroids are situated in the outer part of the belt. Ordinary chondrites rest between them [34]. This location pattern is not surprising; in fact, enstatite chondrites are the most reduced chondrites and must have formed at higher temperatures in the solar nebula, that is, closer to the Sun. In contrast, carbonaceous chondrites have the highest content of volatile elements and must have accreted in cooler regions located farther from the Sun.
Recently, we have had a close-up view of some asteroids and, particularly, of their surface appearance. In 1999, the deep Space 1 mission photographed asteroid Braille. In 1991 and 1993, on its way to Jupiter, the Galileo spacecraft photographed Gaspra, Ida, and its tiny moon, Dactyl, respectively. In 1994, the Hubble Space Telescope mapped Vesta. In 1997 and 1999, the NEAR mission imaged Mathilde and Eros, respectively. Unlike most asteroids, the surface of Vesta, the third largest known asteroid, is significantly heterogeneous, with both dark and light hemispheres. From images taken with the Hubble Space Telescope's Wide Field Planetary Camera, which shows surface details as small as 50 km in diameter, it has been verified that all of Vesta's surface is igneous, indicating that either the entire surface was once melted or that lava-flows once completely covered its surface. In contrast to Vesta, all other asteroids that have been mapped have a dark appearance and chondritic surfaces. Gaspra and Ida, for example, are small type-S objects, their longest dimension being 18 and 56 km, respectively. Both asteroids show evidence of space weathering in the form of an old regolith. Mathilde is the largest asteroid (about 60 km in diameter) ever imaged at high resolution and belongs to the type-C objects. Carbonaceous chondrites are the only meteorites that escaped strong metamorphism and, consequently, offer us the opportunity to explore in detail the characteristics of the organic material that reached the Earth from space before life appeared [13]. Note that, in contrast to the relative scarcity of carbonaceous chondrites finds and falls, type-C asteroids, from which carbonaceous chondrites probably derive, represent about 75% of the known asteroids [34].
The organic richness of carbonaceous chondrites
Organic compounds in carbonaceous chondrites were first observed in 1834 by Berzelius in the Alais carbonaceous chondrite, which fell in France in 1806 [5]. In 1868, Berthelot conducted the first analysis with a sample of Orgueil [4]. Since then, hundreds of investigations have been performed with increasingly sophisticated techniques on several meteorites [45]. Qualitatively and quantitatively analyzing organic molecules in meteorites is an extremely difficult task, as the amounts of some of these compounds are similar to those that would be transferred to the meteorite by just a few fingerprints [40]. Chromatographic and mass spectrometric techniques have, therefore, been widely used with great success. The most valuable information has come from the accurate analysis of the interior of carbonaceous chondrites that were recovered shortly after their fall, that is, that did not experienced extensive terrestrial weathering. In that context, "organized elements" described in the early 1960s in the carbonaceous chondrites Orgueil and Ivuna were initially considered to be evidence that primitive microorganisms were carried to the Earth by meteorites, but were later shown to be contamination products [13]. Also, it has been highly debated whether the Martian meteorite ALH84001, found in Antarctica (there are about 30 Martian meteorites, recognized by their distinctive oxygen isotopic composition and their content of Martian atmospheric gases, trapped in shock-produced glass pockets [34]), contains fossil remains of a past Martian biota or not.
Mixtures of clays, or phyllosilicates, comprise the dominant minerals (50 to 80%) in CI and CM chondrites, and a minor proportion of some CV carbonaceous chondrites. These hydrous minerals resemble some terrestrial clays and were mostly formed as a result of low-temperature aqueous alteration of pre-existing assemblages of anhydrous minerals in asteroids [19]. As an example, Fig. 3 shows the interior of the ALH84034 CM chondrite. Its petrologic analysis indicates that it suffered from severe aqueous alteration on its parent body; both matrix and chondrules had been transformed into clays. Matrix is possibly the least-understood major component of carbonaceous chondrites, but organic matter, which has been intensely studied, is most concentrated in those meteorites, and especially in those portions of the meteorites that experienced the most intense aqueous alteration. In situ textural studies aimed at determining the exact location of the organic matter are still difficult [41]; therefore, its chemical composition is usually studied after chemical extraction from the meteorite.
Refractory organic material
The organic matter in carbonaceous chondrites occurs in multiple forms. A macromolecular material that is extremely fine in grain size, centered at about 10 nm, constitutes the major organic-carbon-bearing phase in all carbonaceous chondrites, representing more than 80% of the total carbon. This complex material is virtually insoluble in solvents and most acids, and appears to be composed of both amorphous and poorly crystalline components, the latter consisting of turbostratic carbon or highly disordered graphite. In the Allende meteorite, a wide range of polycyclic aromatic hydrocarbons (PAHs) with extensively alkylated rings have been observed to be heterogeneously distributed within the matrix [11]. Major species include naphthalene, phenanthrene, anthracene, and their alkyl-substituted derivatives, as well as a wide range of heavier PAHs [48]. The elemental composition of the macromolecular material is about C100H60N7O12S2 [22]. Some fractions of this insoluble material serve as hosts for a variety of distinct noble gases, including several with isotopic compositions that can only be explained in terms of interstellar processes.
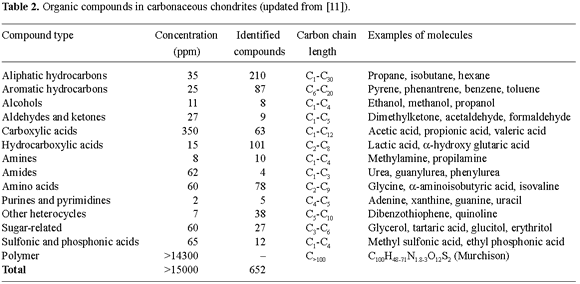
Well-defined organic molecules
Solvent-extractable organic molecules in Murchison are distributed among a large variety of compound families with characteristic functional groups (Table 2). Hundreds of individual organic molecules have been found, and surely many more will be identified in the future. Although these organic compounds comprise less than 0.1% of the total mass of Murchison, their structural and functional diversity are particularly intriguing. Amino acids, the building blocks of proteins, and all of the organic bases in nucleic acids, the fundamental carriers of hereditary information for life as we know it, have been found, for example, in various carbonaceous chondrites. Even if life forms were not carried into the Earth in meteorites, the basic building blocks of life might have been.
Some molecules can assume two mirror image forms, the so-called optical enantiomers. Abiotically produced substances contain, in most cases, equal amounts of the two forms, which results in optical inactivity, whereas in biological synthesis one form is preferred over the other. While most amino acids and carboxylic acids have optical enantiomers, in Murchison, the majority of these substances do not have optical activity, indicating an abiotic mechanism for their formation. However, the search for homochiral substances in branched α-amino acids, which are not present in terrestrial proteins, revealed that L-enantiomers predominate slightly over D-enantiomers [12]. The origin of optical activity in biological organisms has, in fact, originated a vigorous scientific debate. During the last decade, a series of rather simple experiments have demonstrated the feasibility of producing optically active compounds from achiral materials [3]. Space technology in Earth orbit is being currently used to investigate whether amino acids and peptides required for the emergence of life can be safely transported to Earth vicinity without chemical degradation and racemization.
Some of the organic molecules discovered in Murchison have been identified in other carbonaceous chondrites as well. Among the meteorites for which data are available, the extractable organic molecules have different relative abundances for the various classes of compounds, suggesting that their occurrence may be related to different processes in their individual parent bodies.
The formation of organic materials
A major question regarding organic matter in carbonaceous chondrites is how primitive this material is: whether it is an interstellar product, a primary condensate from the solar nebula, or a secondary product, introduced into the meteorite during residence in its parent body. We will start this discussion with the study of the evolution of the carbon atom in the solar nebula since one of the few things that we definitely know is that life is based on carbon. Thermodynamics states that carbon monoxide is the stable form of carbon found in the solar nebula at high temperatures, but it becomes less stable on cooling and should transform to methane below 600 K if a pressure of 10-5 atm is assumed. However, methane has a condensation temperature of less than 100 K at this pressure, and its production is kinetically sluggish, so further cooling to 525 K would lead to the CO disproportion reaction into graphite and CO2 [28]. But if these reactions had taken place as described above, there should be no organic molecules anywhere in the solar system. How can we solve this paradox? By taking into account non-equilibrium processes. Two models have been well studied, the Fischer-Tropsch type (FTT) processes and the Miller-Urey (MU) synthesis, both suggested by chemist Harold Urey [44]. In the FTT process, the production of organic compounds takes place through the hydrogenation of carbon monoxide on the surface of active grains, such as iron particles or iron-nickel alloys (kamacite). This model has received support from theoretical work [15,26] as well as laboratory simulations [16,29,30,32,33], although evaluation of the catalytic role of active grains in the formation of organic compounds under nebular conditions is complicated due to the lack of knowledge on real grain-surface properties. The MU synthesis involves the production and recombination of radical and ionic species in a reduced gas atmosphere under the influence of one or more of several possible energy sources (electric discharge, ultraviolet radiation, galactic rays, etc.), followed by secondary reactions in an aqueous phase. This process is usually assumed to have occurred on the surface of asteroids, although the maintenance of an appropriate atmosphere seems difficult to achieve [44].
The exact nature of the processes responsible for the existence of organic matter in meteorites and the locations where they occurred remain to be clearly established. Gas-grain processes requiring solid surfaces with catalytic properties, electric discharges, and multiple gas-phase reactions could have taken place both in the solar nebula and on asteroids as well as in the interstellar medium. Isotopic studies have revealed that large differences exist in the D/H, 13C/12C, 15N/14N, and 33S/32S ratios associated with different organic compounds in carbonaceous chondrites [46], adding evidence that more than one source region and production mechanism are necessary to account for their occurrence. Laboratory simulations are essential in order to decipher the role of each type of process. Hydrocarbons have been synthesized under nebular conditions by FTT reactions from CO and H2 gaseous mixtures over kamacite grains [32,33], and amino acids have been produced with excellent results through MU reactions [42]. Also, the immediate precursors of amino acids in the MU synthesis-aldehydes, hydrocyanic acid, ammonia and water-have been detected in space by radioastronomy [11].
The interstellar heritage
Laboratory FTT and MU reactions can produce many of the organic compounds discussed above. However, none of these processes can produce D enrichments as large as those recognized in the organic acids, amino acids and macromolecular carbon of the carbonaceous chondrites. The existence of macromolecular organic material, similar in many respects to that present in carbonaceous chondrites, in interstellar space, is universally accepted, since the absorption and emission of light by grains in interstellar space are entirely consistent with the presence there of complex carbon compounds. Probably interstellar grains have silicate cores mantled by complex carbon compounds resulting from radiation processing [44]. Carbonaceous chondrites could incorporate interstellar macromolecular organic materials or their precursors, which in turn could be thermally and chemically reprocessed in the solar nebula to varying degrees.
One of the most astonishing findings in cosmochemistry has been the discovery of presolar dust grains in carbonaceous chondrites, which have been extracted and subjected to close scrutiny in the laboratory. They are micrometer-sized mineral grains that existed as part of interstellar dust as a result of nuclear reactions in dying stars and exploding stars, such as supernovae or giant stars [18], prior to the formation of the Sun and the solar system. Their presolar nature is revealed in the relative abundances of the isotopes of some elements, which differ from those known in all solar system materials [2]. The first interstellar grains were discovered in Murchison in 1987 as minuscule diamonds only a few nanometers across. Since then, several other types of presolar grains have been found, such as silicon carbide, graphite, aluminum oxide, spinel and silicon nitride, in concentrations ranging from 2 parts per billion to 1000 parts per million [34].
The accepted mechanism for the formation of interstellar molecules is based on ion-molecule reactions that, because of the lack of activation energy barriers, can take place rapidly at low temperatures (10 K in interstellar clouds). Ions are originally produced by cosmic rays (cr). The most important process is the ionization of molecular hydrogen: H2 + cr → H2+ + e-. In dark interstellar clouds with abundant H2, this is followed by: H2+ + H2→ H3+ + H. The reason for D enrichment in organic molecules of interstellar origin is that ion-molecule exchange reactions are exothermic, favoring the formation of deuterated molecules: H3+ + HD → H2D+ + H2, H2D+ + CO → DCO+ + H2, etc. The length and complexity of molecules that can be obtained by gas-phase ion-molecule reactions is still unknown but there is certainly a limit. However, condensation of interstellar molecules onto dust grains and subsequent processing and further synthesis on grain surfaces could result in the development of more complex organic species [47].
Influence of asteroidal environment
It is widely accepted that significant amounts of meteoritic organic matter, or its precursor materials, were synthesized in interstellar and nebular environments. However, the final organic molecular architecture of carbonaceous chondrites was strongly determined by the effects of hydrothermal alteration on their parent bodies. The influence that the asteroidal environment appears to exert on the final constitution of meteoritic organic matter has been well demonstrated by laboratory experiments [31]. In terrestrial systems, clay minerals adsorb organic molecules, and this property has led to a newly proposed mechanism for the formation of high-molecular-weight sedimentary organic matter [41]. Similarly, it is probable that, during hydrothermal processing on the carbonaceous chondrite parent bodies, there was adsorption of organic matter between clay layers, leading to their partial oxidation and condensation into larger organic networks and, eventually, giving rise to the macromolecular material that dominates the organic content of carbonaceous chondrites [25,43].
The discovery that meteoritic organic compounds may be trapped and protected within a clay-mineral matrix has strong implications for our understanding of prebiotic molecular evolution in the early solar system. Clay minerals could tra
To summarize, four different synthetic routes have been proposed to account for the large number of distinct organic species present in carbonaceous chondrites. They are: (i) interstellar ion-molecule reactions, (ii) Fischer-Tropsch-type catalytic processes, (iii) Miller-Urey-type reactions, and (iv) hydrothermal transformation mediated by clays. These four end-member models are located in different environments and use different starting materials. But none of the synthetic routes alone is consistent with the composition and isotopic data of the organic matter analyzed in carbonaceous chondrites, and one of them, the MU process, is difficult to reconcile with meteorite petrology. It is necessary to combine more than one process in more than one location.
Meteorites contain secreted within them the oldest and most remote known materials as well as an impressive array of organic molecules. A fascinating aspect of this organic matter is that some may be precursors of life. Answering questions such as what might be the product of chemical evolution of such organic materials and when and where they originated awaits the development of yet more precise analytical techniques and procedures than exist today.
Acknowledgements. Support from the Spanish Ministry of Science and Technology (Ramón y Cajal Research Program) and DURSI (Autonomous Government of Catalonia) is acknowledged.
References
1. Anders E (1989) Pre-biotic organic matter from comets and asteroids. Nature 342:255-257 [ Links ]
2. Anders E, Zinner E (1993) Interstellar grains in primitive meteorites: diamond, silicon carbide and graphite. Meteoritics 128:490-514 [ Links ]
3. Avalos M, Babiano R, Cintas P, Jiménez JL, Palacios JC (2000) Chiral autocatalysis: where stereochemistry meets the origin of life. Chem Commun, pp 887-892 [ Links ]
4. Berthelot MPE (1868) Sur la matière charbonneuse des météorites. Comptes Rendus Hebd Seances Acad Sci 67:849 [ Links ]
5. Berzelius JJ (1834) Ueber Meteorsteine. Ann Phys Chem 33:113-148 [ Links ]
6. Brown PG, Hildebrand AR, Zolensky ME, Grady M, Clayton RN, Mayeda TK, Tagliaferri E, Spalding R, MacRae ND, Hoffman EL, Mittlefehldt DW, Wacker JF, Bird JA, Campbell MD, Carpenter R, Gingerich H, Glatiotis M, Greiner E, Mazur MJ, McCausland PJA, Plotkin H, Rubak-Mazur T (2000) The fall, recovery, orbit, and composition of the Tagish Lake meteorite: A new type of carbonaceous chondrite. Science 290:320-325 [ Links ]
7. Buseck PR, Hua X (1993) Matrices of carbonaceous chondrite meteorites. Annu Rev Earth Planet Sci 21:255-305 [ Links ]
8. Ceplecha Z (1977) Meteoroid populations and orbits. In: Delsemme AH (ed) Comets, asteroids, meteorites. University of Toledo, Ohio, pp 143-152 [ Links ]
9. Chamberlin TC, Chamberlin RT (1908) Early terrestrial conditions that may have favored organic synthesis. Science 28:897-911 [ Links ]
10. Chyba CF, Sagan C (1992) Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origin of life. Nature 355:125-132 [ Links ]
11. Cronin JR, Chang S (1993) Organic matter in meteorites: molecular and isotopic analyses of the Murchison meteorite. In: Greenberg JM (ed) The chemistry of life's origins. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 209-258 [ Links ]
12. Cronin JR, Pizzarello S (1997) Enantiomeric excesses in meteoritic amino acids. Science 275:951-955 [ Links ]
13. Cronin JR, Pizzarello S, Cruikshank DP (1988) Organic matter in carbonaceous chondrites, planetary satellites, asteroids and comets. In: Kerridge JF, Matthews MS (eds) Meteorites and the early solar system. The University of Arizona Press, Tucson, AZ, pp 819-857 [ Links ]
14. Delsemme A (1984) The cometary connection with prebiotic chemistry. Origins Life 14:51-60 [ Links ]
15. Fegley B Jr, Prinn RG (1988) Solar nebula chemistry: implications for volatiles in the solar system. In: Weaver HA, Danly L (eds) The formation and evolution of planetary systems. Cambridge University Press, Cambridge, UK, pp 171-211 [ Links ]
16. Ferrante RF, Moore MH, Nuth III JA, Smith T (2000) Laboratory studies of catalysis of CO to organics on grain analogs. Icarus 145:297-300 [ Links ]
17. Gaffey MJ, Burbine TH, Binzel RP (1993) Asteroid spectroscopy: Progress and perspectives. Meteoritics 28:161-18 [ Links ]
18. Glanz J (1996) Dust grains bring long-lost stars into the laboratory. Science 274:1078-1079 [ Links ]
19. Grimm RE, McSween HY (1989) Water and the thermal evolution of carbonaceous chondrite parent bodies. Icarus 82:244-280 [ Links ]
20. Grossman L (1972) Condensation in the primitive solar nebula. Geochim Cosmochim Acta 36:597-619 [ Links ]
21. Haldane JBS (1929) The origin of life. The Rationalist Annual. Reprinted in Deamer DW, Fleischaker GR (ed) (1994) Origins of life: the central concepts. Jones and Barlett, Boston, MA, pp 73-81 [ Links ]
22. Hayatsu R, Anders E (1981) Organic compounds in meteorites and their origins. Topics Current Chemistry 99:1-37 [ Links ]
23. Holland HD (1984) The chemical evolution of the atmosphere and oceans. Princeton University Press, Princeton, PA [ Links ]
24. Kasting J, Ackerman TP (1986) Climatic consequences of very high carbon dioxide levels in the Earth's early atmosphere. Science 234:1383-1385 [ Links ]
25. Kitajima F, Nakamura T, Takaoka N, Murae T (2002) Evaluating the thermal metamorphism of CM chondrites by using the pyrolytic behavior of carbonaceous macromolecular matter. Geochim Cosmochim Acta 66:163-172 [ Links ]
26. Kress M, Tielens AGGM (2001) The role of Fischer-Tropsch catalysis in solar nebula chemistry. Meteorit Planet Sci 36:75-91 [ Links ]
27. Lee T, Papanastassiou DA, Wasserburg GJ (1976) Demonstration of 26Mg excess in Allende and evidence for 26Al. Geophys Res Lett 3:109-112 [ Links ]
28. Lewis RS, Prinn RG (1980) Kinetic inhibition of CO and N2 reduction in the solar nebula. Astrophys J 238:357-364 [ Links ]
29. Llorca J (1999) Hydrocarbon synthesis in cometary grains. Phys Chem Earth 24:591-595 [ Links ]
30. Llorca J (2002) Are organic molecules produced by nebular Fischer-Tropsch processes preserved in comets? Adv Space Res 30:1469-1472 [ Links ]
31. Llorca J (2002) Hydrothermal processing of organic molecules produced by gas-grain reactions under nebular conditions. ESA Special Publ 518:475-476 [ Links ]
32. Llorca J, Casanova I (1998) Formation of carbides and hydrocarbons in chondritic interplanetary dust particles: A laboratory study. Meteorit Planet Sci 33:243-251 [ Links ]
33. Llorca J, Casanova I (2000) Reaction between H2, CO and H2S over Fe, Ni metal in the solar nebula. Experimental evidence for the formation of sulfur-bearing organic molecules and sulfides. Meteorit Planet Sci 35:841-848 [ Links ]
34. McSween HJr (1989) Meteorites and their parent planets. Cambridge University Press, Cambridge, UK [ Links ]
35. Miller SL (1953) A production of amino acids under possible primitive Earth conditions. Science 117:528-529 [ Links ]
36. Miller SL, Urey HC (1959) Organic compound synthesis on the primitive Earth. Science 130:245-251 [ Links ]
37. Newsom H, Taylor SR (1989) Geochemical implications of the formation of the Moon by a single giant impact. Nature 338:29-34 [ Links ]
38. Oparin AI (1924) Proiskhodenie Zhizny. Moscovky Rabotchii, Mockba. English translation in Deamer DW, Fleischaker GR (1994) Origins of life: the central concepts. Jones and Barlett, Boston, MA, pp 31-71 [ Links ]
39. Oró J (1961) Comets and the formation of biochemical compounds on the primitive Earth. Nature 190:389-390 [ Links ]
40. Oró J, Skewes HB (1965) Free amino acids on human fingers: the question of contamination in microanalysis. Nature 207:1042-1045 [ Links ]
41. Pearson VK, Sephton MA, Kearsley AT, Bland BA, Franchi IA, Gilmour I (2002) Clay mineral-organic matter relationships in the early solar system. Meteorit Planet Sci 37:1829-1833 [ Links ]
42. Peltzer ET, Bada JL, Schlesinger G, Miller SL (1984) The chemical conditions on the parent body of the Murchison meteorite: Some conclusions based on amino, hydroxy and dicarboxylic acids. Adv Space Res 4:69-74 [ Links ]
43. Quirico E, Raynal PI, Bourot-Denise M (2003) Metamorphic grade of organic matter in six unequilibrated ordinary chondrites. Meteorit Planet Sci 38:795-811 [ Links ]
44. Sandford SA (1996) The inventory of interstellar materials available for the formation of the solar system. Meteorit Planet Sci 31:449-476 [ Links ]
45. Sephton MA (2002) Organic compounds in carbonaceous chondrites. Nat Prod Rep 19:292-311 [ Links ]
46. Sephton MA, Verchovsky AB, Bland PA, Gilmour I, Grady MM, Wright Ip (2003) Investigating the variations in carbon and nitrogen isotopes in carbonaceous chondrites. Geochim Cosmochim Acta 67:2093-2108 [ Links ]
47. Strazzulla G (1997) Ion bombardment of comets. In: Pendleton YJ, Tielens AGGM (ed) From stardust to planetesimals. ASP Conf Ser 122:423-433 [ Links ]
48. Zenobi R, Philippoz JM, Buseck PR, Zare RN (1989) Spatially resolved organic analysis of the Allende meteorite. Science 246:1026-1029 [ Links ]