Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Pediatría Atención Primaria
versión impresa ISSN 1139-7632
Rev Pediatr Aten Primaria vol.15 no.58 Madrid abr./jun. 2013
https://dx.doi.org/10.4321/S1139-76322013000200002
Nebulized 3% hypertonic saline solution in hospitalized infants with acute bronchiolitis: case-control study on the utility and effectiveness
Estudio sobre la eficacia y utilidad de la solución salina hipertónica al 3% en la bronquiolitis aguda del lactante hospitalizado
R. Martín Martína, G. Yep Chullenb, M. Sánchez Bayleb, E. Villalobos Pintob, P. Flores Pérezb
aPaediatrician. Centro de Salud Reina Victoria, Madrid. Spain.
bPaediatrics Department, Hospital Infantil del Niño Jesús, Madrid. Spain.
ABSTRACT
Objective: to study the utility of nebulized 3% hypertonic saline solution (HSS) in hospitalized infants with acute bronchiolitis.
Patients and methods: case-control studies accomplished on 639 patients of age less than 7 months old and hospitalized with the diagnosis of acute bronchiolitis, first episode, during 3 consecutive seasons in a pediatric department in Madrid. The patients who received 0.9% saline solution (FSS), with or without medication, during the 2 first seasons were considered the control group and the patients who received, the last season period, nebulized 3% hypertonic saline solution were considered the cases group. The days of hospitalization and the hours of oxygen therapy were used as the result measurement.
Results: from the total of the studied children, 460 received 0.9% saline solution and 179 received 3% hypertonic saline solution. In the group receiving FSS the average stay in hospital was 5.16 days (95% confidence interval [95% CI] 4.78-5.56) and the average time of oxygen therapy was 57.34 hours (95% CI 52.93-61.75) opposite to 4.90 days (95% CI 4.64-5.07) and 67.53 hours (95% CI 60.36-74.69) respectively in the group that received HSS. There was no significant difference between the groups. The patients who received FSS and were positive for VRS and also patients less than 3 months old, showed a significant reduction in the oxygen therapy hours (p= 0.004 and p= 0.007 respectively).
Conclusions: results show that 3% hypertonic saline solution has not been effective in reducing hospital stay or length of oxygen therapy in patients with acute bronchiolitis; but nebulized 0,9% saline solution in children with age <3 months and positive study of respiratory syncitial virus in nasopharyngeal aspirate showed a reduced need of hours of oxygen.
Key words: Acute bronchiolitis. Treatment.
RESUMEN
Objetivo: estudiar la utilidad de la solución salina hipertónica (SSH) al 3% inhalada en el tratamiento de la bronquiolitis aguda (BA) del lactante hospitalizado.
Pacientes y métodos: estudio de casos y controles realizado con 639 pacientes de edad inferior a siete meses e ingresados con diagnóstico de BA, primer episodio, durante tres periodos estacionales consecutivos, en la sección de lactantes de un hospital pediátrico de Madrid (España). Los pacientes que recibieron como tratamiento, durante los dos primeros periodos estacionales, suero salino fisiológico (SSF) inhalado con o sin medicación se consideraron el grupo control y los pacientes que recibieron, durante el tercer periodo estacional, suero salino hipertónico al 3% inhalado con o sin medicación se consideraron como casos. Los días de hospitalización y las horas de oxigenoterapia fueron utilizados como medidas de resultado.
Resultados: de la totalidad de los niños estudiados, 460 recibieron SSF inhalado, y 179 recibieron SSH al 3%. En el grupo que recibió SSF, la estancia media en el hospital fue de 5,16 días (intervalo de confianza del 95% [IC 95%]: 4,78-5,56) y el tiempo medio de oxigenoterapia fue de 57,34 (IC 95%: 52,93-61,75) frente a 4,90 días (IC 95%: 4,64-5,07) y 67,53 horas (IC 95%: 60,36-74,69), respectivamente, en el grupo tratado con SSH. Estos resultados no alcanzan significación estadística. Los pacientes con estudio positivo de virus respiratorio sincitial (VRS) en aspirado nasofaríngeo y que recibieron SSF necesitaron menos horas de oxígeno de manera significativa (p=0,004), así como aquellos que tenían edad <3 meses (p=0,007).
Conclusiones: los resultados obtenidos muestran que la SSH al 3% inhalada no resulta eficaz para reducir la estancia hospitalaria ni el tiempo de oxigenoterapia en los pacientes con BA; además, en los niños menores de tres meses y con estudio positivo de VRS en aspirado nasofaríngeo la aplicación de SSF inhalado consiguió una necesidad menor de horas de oxígeno.
Palabras clave: Bronquiolitis aguda. Tratamiento.
Introduction
Acute bronchiolitis (AB) is the most common lower respiratory tract infection in children younger than a year, with the youngest infants requiring hospitalisation most frequently and being subjected to therapeutic interventions and diagnostic tests whose efficacy and usefulness are not sufficiently proven1.
AB may be one of the most widely studied pathologies in children, with numerous clinical practice guidelines and expert group recommendations addressing the condition2,3, yet despite all the published information there is no consensus on how to provide treatment for this group of patients. The lag between clinical practise and scientific evidence leads to a high and unjustified use of social and economic resources4,5.
AB is characterised by an acute inflammation of the terminal bronchioles, with airway oedema and mucus plugging being the predominant pathological features, which is why any therapeutic approach that can decrease these alterations and improve secretion clearance can be beneficial6.
Hypertonic saline solutions (HSS) are composed of sodium chloride dissolved in distilled water. More specifically, 3% saline is produced by making a 20% solution of sodium chloride in physiological saline solution (PSS)7, and its inhalation has been demonstrated to improve mucociliary clearance in vivo and in vitro in diseases such as cystic fibrosis, asthma, and bronchiectasis. Thus far, oxygen therapy is the only treatment that has been shown to improve the clinical course of AB, which is why the management of these patients is based on general supportive care measures8.
The goal of this study is to examine the efficacy of 3% HSS inhalation for the treatment of the first episode of acute bronchiolitis in a group of infants admitted to the Hospital Infantil del Niño Jesús in Madrid.
Patients and methods
This is a case-control study, carried out with 639 patients of ages below seven months who were admitted with a diagnosis of AB, first episode, to the infant ward of the Hospital Infantil del Niño Jesús of Madrid, during the winter months of years 2007-2008, 2008-2009, and 2009-2010.
We did not perform sample size calculations because we considered that the number of admissions with an AB diagnosis in the selected period was within the expected range, and also due to the unpredictability of the incidence of this disease.
Children were diagnosed with AB if they had a history of preceding viral upper respiratory tract infection and a clinical presentation with respiratory distress and wheezing or crackles on chest auscultation (McConnochie criteria). The children that presented at least one of the following symptoms during the emergency room visit were admitted to the hospital: "toxic" appearance, history of apnoea/cyanosis, respiratory rate >60/minute, and oxygen saturation <94%.
We excluded from the study children with chronic respiratory problems or cardiopathies, and those children who presented with critical AB illness requiring admission to the intensive care unit.
To perform the study, during the 2007-2008 and 2008-2009 seasons the patients were given nebulised PSS with or without medication, with these patients constituting the controls; and during the 2009-2010 season, patients were given 3% HSS alone or in combination with medication, with these patients constituting the cases.
The treatment consisted of administering 3 cc of saline solution with a standard nebulizer along with oxygen every eight hours if this was the sole treatment, and every four to six hours if it was given in combination with drugs.
The outcome measures used in this study were the duration of the hospital stay in days and the hours of oxygen therapy received.
The criterion used to discontinue oxygen therapy was achieving an oxygen saturation level ≥94%. Oxygen saturation levels were recorded by the nursing staff every four hours.
The criteria for discharge were not having a fever, a good general health status, tolerating oral feeding, and not requiring oxygen therapy.
We performed the statistical analysis using the commercial software SPSS® 11.0. We expressed the basic data in means and standard deviations for quantitative variables, and in frequencies and percentages in the case of qualitative variables. We calculated the 95% confidence intervals (95% CI).
We used the Mann-Whitney U test for comparing quantitative variables after finding that they did not fit a normal distribution (Kolmogorov-Smirnov test). The comparison of qualitative variables was done using the chi-squared test. We considered the results statistically significant for p values below 0.05.
Results
The total number of patients admitted with an AB diagnosis and younger than seven months during the 2007-2008, 2008-2009, and 2009-2010 seasons included in this study was 639, of whom 237 (37.08%) were boys, and the rest girls. In the first two seasons, 460 patients were treated with nebulised PSS alone or in combination with bronchodilator drugs, and they constituted the controls; and in the last season, 179 patients were given 3% HSS alone or in combination with bronchodilator drugs, and they constituted the cases.
The age range of the patients was ten days to 6.5 months, and since the youngest patients suffer the most severe episodes of AB, we divided the patients into two groups, one age <3 months and another age ≥3 months.
Table 1 shows the general characteristics of the patients and the comparisons between the two groups according to the treatment they received, and we saw that there were no significant differences between them.

Table 2 shows the outcomes of all the studied patients according to the treatment received, for which we found no significant difference in the duration of the hospitalisation in days, but did find a difference in the number of hours of oxygen therapy required, with the PSS group requiring fewer hours (p=0.001).
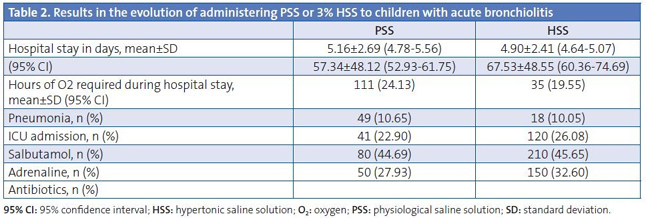
Table 3 shows the results obtained in relation to the presence or absence of respiratory syncytial virus (RSV) in the nasopharyngeal aspirates. We found no significant difference in the number of days the patients were hospitalised, but we did find a difference in the required hours of oxygen therapy, with RSV+ patients treated with PSS requiring fewer hours (p=0.004).
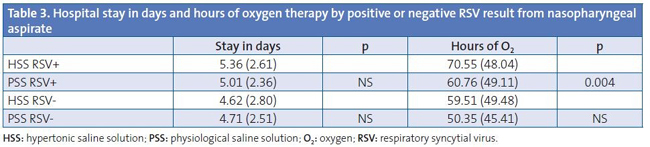
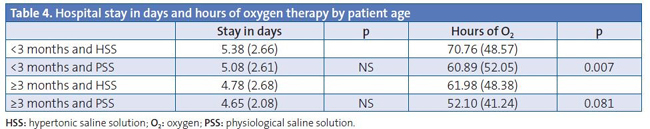
Table 4 analyses the results in relation to the age of the patients (below or equal/above three months); in this instance, we found a decreased need for oxygen therapy in patients <3 months treated with PSS (p=0.007). When it came to the hours of oxygen therapy in patients ≥3 months, the result we obtained was right on the edge of statistical significance (p=0.081).
Discussion
This study contributes an assessment of treatment with nebulised 3% HSS administered to infants hospitalised with a first episode of AB, using the length of hospitalisation and the hours of oxygen therapy received as the outcome measures.
We have known for a while that HSS increases mucociliary clearance in normal patients9 and that its use is useful and safe as a therapeutic strategy in diseases with defective mucociliary clearance such as asthma, bronchiectasis, and cystic fibrosis11.
The potential beneficial effects of hypertonic saline solution may be due to its theoretical ability to lower the viscosity and elasticity of the mucous gel: breaking the ionic bonds in the mucus; causing an osmotic flow of water toward the inside of the mucous gel, rehydrating it and improving its rheology; stimulating ciliary beating by the release of prostaglandins; reducing the oedema of the airway wall by absorbing water from the mucosa and submucosa; or lastly by inducing coughing and mucus production12.
The literature we reviewed included studies done with hospitalised patients and studies with patients that sought emergency room care but were not admitted to the hospital. The outcome measures selected in each case were different, and consequently the results obtained from hospitalised patients cannot be extrapolated to outpatient services and vice versa, which means that there is a setting-related bias that, of course, is also present in our study.
When it comes to the duration of hospital stays, the average length of hospitalisation due to AB in the literature is of 3 to 5 days, and our results conform to the literature on this point with a mean of 4.9 days in children who were given HSS and of 5.16 days in children given PSS. In our study, the administration of 3% HSS did not succeed in decreasing hospitalisation length significantly, and neither did the administration of PSS. The Cochrane review that we consulted13 included three hospital studies14-16in which the authors presented statistically significant results, with a 0.9 day decrease in the duration of the hospital stay following administration of 3% HSS; however, it is obvious that this result is not significant for clinical practise. The Cochrane review itself has been the subject of critical evaluation by other authors17-20, as establishing the therapeutic function of HSS has significant clinical implications. In their studies, Luo Z, et al.21,22 conclude that the administration of 3% HSS is safe and efficacious, significantly decreasing the length of hospitalisation of the patients under study.
The children included in this study required oxygen therapy during their hospital stay, and the hours of therapy required were another outcome measure. We found that the need for oxygen therapy was significantly reduced in the group of children younger than 3 months who were given nebulised PSS; furthermore, the children whose nasopharyngeal aspirates tested positive for RSV and who were given nebulised PSS also required fewer hours of oxygen therapy. 3% HSS did not show any benefits in any case. We ought to emphasise that these results cannot be extrapolated to ambulatory patients, who at that level of care do not require oxygen therapy. We analysed the RSV+ group separately to see whether the efficacy of the treatment varied depending on the aetiology of AB. We do not know how to account for these results, which pertain to age and to RSV+ status, and we have found no references to this in the reviewed literature.
In the studies done with ambulatory patients, the outcome measures used to assess the efficacy of the treatment consisted in evaluating the improvement of symptoms following its application, and in quantifying the reduction in hospital admissions. Most of the studies we reviewed23-25 did not find a significant difference between the administration of PSS and of 3% HSS; Al-Ansari, et al.26 evaluated the use of saline solutions at 5, 3, and 0.9% and found the 5% solution more efficacious. The Cochrane review includes a study27 with ambulatory patients whose authors did find statistically significant beneficial effects to the administration of 3% HSS.
We did not find any adverse effects to the treatment, and in this regard all authors agree in stating that the administration of 3% HSS alone28 or in combination with bronchodilators is harmless, safe, and cheap.
One limitation in our study was that the patients were not randomly assigned to treatment and control groups.
To conclude, we would like to emphasise that the administration of 3% HSS alone or combined with medication to the patients admitted to our hospital with AB did not prove to be efficacious in decreasing the length of hospitalisation nor the duration of oxygen therapy. Considering the prevalence of AB, and its social and economic repercussions, we should emphasise the need to carry out studies on this subject in the future.
Conflict of interests
The authors declare that they had no conflict of interests when it came to preparing and publishing this paper.
Acronyms: 95% CI: 95% confidence interval • AB: acute bronchiolitis • HSS: hypertonic saline solution • PSS: physiological saline solution • RSV: respiratory syncytial virus.
![]() Correspondence:
Correspondence:
Raquel Martín Martín
raquelmartin333@hotmail.com
Bibliography
1. González de Dios J, Ochoa Sangrador C, Grupo Investigador del Proyecto aBREVIADo (BRonquiolitis-Estudio de Variabilidad Idoneidad y Adecuación). Estudio de variabilidad en el abordaje de la bronquiolitis aguda en España en relación con la edad de los pacientes. An Pediatr (Barc). 2010;72:4-18. [ Links ]
2. Simó Nebot M, Claret Teruel G, Luaces Cubells C, Estrada Sabadell MD, Pou Fernández J. Guía de práctica clínica sobre la bronquiolitis aguda: recomendaciones para la práctica clínica. An Pediatr (Barc). 2010;73:208.e1-10. [ Links ]
3. González de Dios J, Ochoa Sangrador C, Grupo de Trabajo (Grupo Investigador, Grupo de Revisión y Panel de Expertos) del Proyecto aBREVIADo (Bronquiolitis-Estudio de Variabilidad, Idoneidad y Adecuación). Recomendaciones de la Conferencia de Consenso de Bronquiolitis aguda en España: de la evidencia a la práctica. Rev Pediatr Aten Primaria. 2010;12 (Supl 19):s107-128. [ Links ]
4. Sánchez Etxaniz J, Benito Fernández J, Mintegi Raso S. Bronquiolitis aguda: ¿por qué no se aplica lo que se publica? Barreras en la transmisión del conocimiento. Rev Pediatr Aten Primaria. 2008;10:23-32. [ Links ]
5. Calogero C, Sly PD. Acute viral bronchiolitis: To treat or not to treat. That is the question. J Pediatr. 2007;151(3):235-37. [ Links ]
6. Mandelberg A, Amirav I. Hypertonic saline or high volume normal saline for viral bronchiolitis: mechanisms and rationale. Pediatr Pulmonology. 2010;45(1):36-40. [ Links ]
7. Borja Urbano G, Pérez Pérez G, Andrés Martín A, Navarro Merino M. Actualización en el manejo de la bronquiolitis. Vox Paediatrica. 2011;XVIII(2):57-67. [ Links ]
8. Pérez Rodríguez MJ, Otheo de Tejada Barasoain E, Ros Pérez P. Bronquiolitis en pediatría: puesta al día. Inf Ter Sist Nac Salud. 2010;34:3-11. [ Links ]
9. Daviskas E, Anderson SD, Gonda I, Eberl S, Meikle S, Seale P, et al. Inhalation of hypertonic saline aerosol enhances mucociliary clearance in asthmatic and healthy subjects. Eur Respir J. 1996;9:725-32. [ Links ]
10. Robinson M, Regnis JA, Bailey DL, King M, Bautovich GJ, Bye PTP. Effect of hypertonic saline, amiloride and cough on muciciliary clearance in patients with cystic fibrosis. Am J Respir Crit Care Med. 1996;153:1503-9. [ Links ]
11. Fraga Betancur DA, Baez JC, Reyes JM, Sussini MA. Uso de soluciones salinas hipertónicas inhaladas para restaurar la hidratación superficial de la vía aérea en pacientes con fibrosis quística. Rev Posgrado de la VI.a Cátedra de Medicina. 2006;164:13-6. [ Links ]
12. González de Dios J, Ochoa Sangrador C, Grupo de Revisión del Proyecto aBREVIADo (Bronquiolitis-Estudio de Variabilidad, Idoneidad y Adecuación). Conferencia de Consenso sobre bronquiolitis aguda (IV): tratamiento de la bronquiolitis aguda: Revisión de la evidencia científica. An Pediatr (Barc). 2010;72:285.e1-42. [ Links ]
13. Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulized hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev. 2008;4:CD006458. [ Links ]
14. Mandelberg A, Tal G, Witzling M, Someck E, Houri S, Balin A, et al. Nebulized 3% hypertonic saline solution treatment in hospitalized infants with viral bronchiolitis. Chest. 2003;123:481-7. [ Links ]
15. Tal G, Cesar K, Oron A, Houri S, Ballin A, Mandelberg A. Hypertonic saline/epinephrine treatment in hospitalized infants with viral bronchiolitis reduces hospitalization stay: 2 years experience. Isr Med Assoc J. 2006;8(3):169-73. [ Links ]
16. Kuzik BA, Al-Qadhi SA, Kent S, Flavin MP, Hopman W, Hotte S, et al. Nebulized hypertonic saline in the treatment of viral bronchiolitis in infants. J Pediatr. 2007;151:266-70. [ Links ]
17. Fernández Rodríguez M, Martín Muñoz P. Los aerosoles con suero salino hipertónico al 3% podrían disminuir la duración de la hospitalización en lactantes con bronquiolitis. Rev Pediatr Aten Primaria. 2008;10:91-5. [ Links ]
18. Balaguer Santamaría A, Buñuel Álvarez JC, González de Dios J. El suero salino hipertónico nebulizado puede disminuir la duración del ingreso hospitalario en lactantes con bronquiolitis aguda. Evid Pediatr. 2009;1:5. [ Links ]
19. Horner D, Jenner R. Nebulised hypertonic saline significantly decreases length of hospital stay and reduces symptoms in children with bronchiolitis. Emerg Med J. 2009;26(7):518-9. [ Links ]
20. Mathew JL. Hypertonic saline nebulization for bronchiolitis. Indian Pediatrics. 2008;45:987-9. [ Links ]
21. Luo Z, Liu E, Luo J, Li S, Zeng F, Yang X, et al. Nebulized hypertonic saline/salbutamol solution treatment in hospitalized children with mild to moderate bronchiolitis. Pediatr Int. 2010;52(2):199-202. [ Links ]
22. Luo Z, Fu Z, Liu E, Xu X, Fu X, Peng D, et al. Nebulized hypertonic saline treatment in hospitalized children with moderate to severe viral bronchiolitis. Clin Microbiol Infect. 2011;17(12):1829-33. [ Links ]
23. Grewal S, Ali S, McConnell DW, Vandermeer B, Klassen TP. A randomized trial of nebulized 3% hypertonic saline with epinephrine in the treatment of acute bronchiolitis in the emergency department. Arch Pediatr Adolesc Med. 2009;163(11):1007-12. [ Links ]
24. Kuzik BA, Flavin MP, Kent S, Zielinski D, Kwan Ch W, Adeleye A, et al. Effect of inhaled hypertonic saline on hospital admission rate in children with viral bronchiolitis: a randomized trial. CJEM. 2010;12(6):477-84. [ Links ]
25. Anil AB, Anil M, Saglam AB, Cetin N, Bal A, Aksu N. High volume normal saline alone is as effective as nebulized salbutamol normal saline, epinephrine-normal saline, and 3% saline in mild bronchiolitis. Pediatr Pulmonol. 2010;45(1):41-7. [ Links ]
26. Al-Ansari K, Sakran M, Davidson BL, El Sayyed R, Mahjoub H, Ibrahim K. Nebulized 5% or 3% hypertonic or 0,9% saline for treating acute bronchiolitis in infants. J Pediatr. 2010;157(4):630-4. [ Links ]
27. Sarrell EM, Tal G, Witzling M, Someck E, Houri S, Cohen HA, et al. Nebulized 3% hypertonic saline solution treatment in ambulatory children with viral bronchiolitis decreases symptoms. Chest. 2002;122(6):2015-20. [ Links ]
28. Ralston S, Hill V, Martinez M. Nebulized hypertonic saline without adjunctive bronchodilators for children with bronchiolitis. Pediatrics. 2010;126:e520-e525. [ Links ]











 texto en
texto en 


