My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Pediatría Atención Primaria
Print version ISSN 1139-7632
Rev Pediatr Aten Primaria vol.18 n.71 Madrid Jul./Sep. 2016
CLINIC CORE. ORIGINAL PAPERS
Unusual clinical findings in an outbreak of scarlet fever
aPediatra. Centro de Salud Vélez-Sur. Unidad de Gestión Clínica de Pediatría y Neonatología Málaga-Este. Axarquía. Málaga. España.
bPediatra. Centro de Salud Vélez-Sur. Unidad de Gestión Clínica de Pediatría y Neonatología Málaga-Este. Axarquía. Málaga. España.
cMédico de Familia. Centro de Salud Vélez-Sur. Unidad de Gestión Clínica Vélez-Málaga Sur. Área de Gestión Sanitaria Este de Málaga. Axarquía. Málaga. España.
Introduction:
scarlet fever is an infectious disease caused by Streptococcus pyogenes that manifests as a typical pharyngoamigdalitis and exanthema. Its diagnosis is usually easy, but atypical cases may go unnoticed.
Patients and methodology:
retrospective descriptive study of pediatric population assigned to a Primary Care center a health centre between 2013/2014. We define the epidemiology, clinical characteristics, microbiological tests, treat ment and appearance of relapses.
Results:
91 cases, resulting in an incidence of 3.2% of which 76 were confirmed microbiologically with a rapid test or culture. The average age was 4,15 years. The main reasons for consultation were "fever and sore throat" and "fever and rash". The most common alterations were pharyingeal hyper emia and petechiae on the palate and in a few patients we found tonsillar exudate. Almost 40% of patients had catarrhal symptoms. 71 patients showed a typical exanthema and 20 of them an atypical one. Most of them were treated with amoxicillin or penicillin for 10 days. 15 patients had recurrence.
Conclusions:
from the data obtained it is important to highlight the large amount of cases, the presence of catarrhal symptoms and the infrequency of tonsillar exudates. It was remarkable the variability of recurrences with findings such as extensive erythroderma, urticaria, macular rashes, atypically placed petechiae and facial and member edema. The rapid test on primary care units allows diagnosis on doubtful cases.
Keywords: antigen detection testing; Exanthema; Streptococcus pyogenes; Children; Scarlet fever.
INTRODUCTION
Scarlet fever is a disease that results from infection group A Streptococcus pyogenes (GAS) strains producing pyrogenic exotoxins (previously known as erythrogenic toxins) in individuals that do not have antibodies against the toxin. It is an infection of the upper respiratory tract that commonly manifests with a characteristic rash.1
Children with untreated scarlet fever spread GAS through saliva droplets from nasal and respiratory tract secretions, although there are documented cases of transmission through food and fomites. Transmission is facilitated by close contact, and thus nurseries, schools and the home are significant settings for transmission.1,3
The incubation period lasts between two and five days. Children usually stop being infectious after 24 hours of appropriate antibiotic treatment, although recent data has shown that 12 hours may be sufficient. Chronic pharyngeal carriers of GAS rarely transmit this bacterium to others.1,4
According to numerous historical texts and some scientific articles, scarlet fever is infrequent in the present. However, multiple sources in the literature have reported an increased number of cases in recent years. Its incidence is cyclical and depends on the prevalence of toxin-producing strains and the immune health of the population, and it is higher in children aged 5 to 15 years, especially the younger set, although it is not rare in other ages.1,5,6
The classical clinical presentation is as follows. The findings of the pharyngeal examination are similar to those of streptococcal pharyngitis. The tongue usually has oedematous papillae with a whitish hue (saburral tongue) that eventually redden (strawberry tongue). Exanthema usually appears 24 to 48 hours from the onset of symptoms, although it can also be the first symptom to develop. It is a diffuse erythematous and papular rash that results in a deep reddening of the skin that blanches with pressure, and the skin becomes coarse (sandpaper skin). It is usually more intense along skin folds (Pastia lines) and spares the nasolabial triangle, which lends a pale appearance to the perioral region (Filatov's sign). After three to four days, the exanthema starts to fade and is followed by light desquamation.1,7
Typical scarlet fever is not difficult to diagnose, but there are atypical presentations with findings that are not easily recognizable and that require a differential diagnosis with other diseases. In unclear cases, the detection of GAS in the pharynx can help the diagnosis.1,7,8,9,10,11
The complications of scarlet fever are the same as those associated with pharyngotonsillitis. Early complications are suppurative, such as otitis media, pharyngeal abscess and sinusitis, and late complications are nonsuppurative, such as glomerulonephritis and rheumatic fever.7
Scarlet fever is a disease that can develop more than once in a lifetime due to the existence of different genetic lineages of GAS that produce different pyrogenic toxins.5,9,11,12,13,14
PATIENTS AND METHODS
We conducted a retrospective descriptive study of cases of scarlet fever diagnosed between September 1, 2013 (week 35 of 2013) and August 31, 2014 (week 35 of 2014).
The study was conducted in the town of Vélez-Málaga (Spain). The population under study consisted of children aged 0 to 13 years included in the two paediatrics caseloads of the Vélez-Sur Health Care Centre. The total number of health care users in this age group, which we obtained from the database of the Public Health System of Andalusia, was 2852, corresponding to the total number of patients for the two caseloads.
We included all patients that received a diagnosis of scarlet fever as cases. We defined confirmed case as a case that fulfilled the clinical criteria (pharyngotonsillitis and exanthema) confirmed by microbiological testing, and probable case as a case in which the diagnosis was based solely on the clinical manifestations.
We searched for cases using the electronic medical records of the Diraya database of the Public Health System of Andalusia.
We analysed the frequency of cases, patient sex, age of onset, distribution of cases per month and week, clinical presentation (signs and symptoms reported during history taking as well as findings of physical examination) and recurrence. We also studied whether microbiological tests were performed and the treatment given.
The most frequently used microbiological test was the OSOM(r) Strep A rapid test on throat swab specimens (Leti Diagnóstica(r)) which, according to several studies, has a sensitivity of 85% to 95%, a specificity of 92% to 98%, a positive predictive value of 80% to 91% and a negative predictive value of 95% to 98%.15,16,17,18 Culture was performed in only a few patients.
RESULTS
We obtained a sample of 91 cases, which corresponded to an incidence of 3.2% in the population under study (91/2852) during the analysed period.
Of the patients that had scarlet fever, 52 were male (57.1%) and 39 female (32.9%). The mean age was 4.15 years, and there were two modes at ages 4 and 5 years (Figure 1). There was a higher frequency of cases in January, February, March, April and May. (Figure 2). The distribution by weeks showed two incidence peaks at weeks 11 and 15 of the year, corresponding to March and April, respectively (Figure 3).
The most frequent reasons for seeking care were "fever and throat pain" (25%) and "fever and skin rash" (23%), while 14% presented with isolated fever and 10% with isolated exanthema (Figure 4).
Table 1 presents the symptoms reported by the patient and/or the caregiver during the history taking. In decreasing order of frequency, they were fever, throat pain, skin rash, nasal secretions and/or cough, vomiting, abdominal pain, headache and pruritus. Other symptoms reported less frequently included constipation, facial swelling, dysuria, ear pain and pain in the extremities.
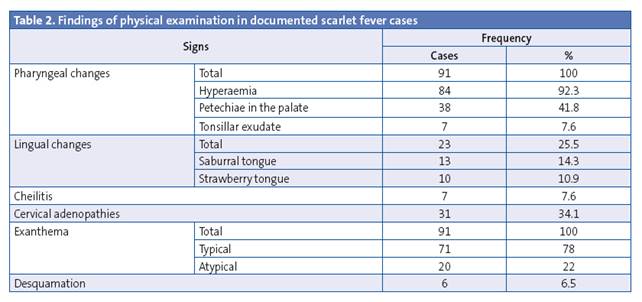
Table 2 summarises the findings of the physical examination. All patients presented with pharyngeal abnormalities and exanthema, which we consider necessary clinical findings for the diagnosis of scarlet fever. The most frequent pharyngeal abnormalities were hyperaemia and petechiae in the palate, while few patients had tonsillar exudate. A typical exanthema (understood as exanthema with the characteristics described in the introduction) was found in 71 patients, while another 20 patients presented with atypical features: five had a smooth macular exanthema, one had plantar-palmar exanthema, one had erythema and swelling in the ear lobes, one extensive erythroderma, and two localised facial erythroderma accompanied by the typical coarse exanthema in the rest of the body, five presented with swelling of the eyelids and/or the face and/or the distal extremities, one with petechiae in the face, neck and axillae, one had two episodes of hives (one of them with sandpaper skin). All patients that had atypical exanthema had a positive microbiological test, which is why they were included in the analysis. Other findings of the examination included abnormalities in the tongue, cheilitis and cervical adenopathies. Desquamation was only documented in six cases. One patient had perianal dermatitis, and both the perianal and the pharyngeal exudates tested positive for streptococcus.
Seventy-six cases were confirmed by microbiological testing (75 by rapid testing and one by culture), and fifteen were probable cases corresponding to patients that had received prior care in the emergency department, where the rapid test was not available, and who were already receiving antibiotic treatment by the time they were seen at the primary care clinic.
The suppurative complications found in our study consisted of acute otitis media in three patients. None of the patients had nonsuppurative complications.
Scarlet fever recurred in fifteen patients: ten patients (11%) experienced recurrences of scarlet fever diagnosed in the period under study (nine had two episodes and one had three) and five patients (5.4%) recurrences of scarlet fever diagnosed before the period under study (one of them had two episodes). One of the patients had four episodes in total, two during the period under study and two in the months that followed.
Patients were treated with antibiotherapy: penicillin in 23 (25.3%); amoxicillin in 60 (65.9%); amoxicillin-clavulanic acid in five (5.5%); josamycin in one patient allergic to amoxicillin; and clindamycin in a patient experiencing a third episode. A single patient with suspected toxic shock that presented with extensive erythroderma was treated with intravenous cloxacillin, clindamycin and amoxicillin-clavulanic acid. The prescribed duration of treatment was ten days, except in a single patient that was treated for only eight days.
DISCUSSION
In Spain, the reporting of scarlet fever cases stopped being mandatory in 1996, so at present we do not have reliable epidemiological data that would serve as a referece.7,9In the United Kingdom, where there is epidemiological surveillance of scarlet fever, the data has shown a cyclical pattern with incidence peaks every four years.
The increased incidence in our population coincided with the increase in the number of cases of scarlet fever above the usual seasonal levels throughout the United Kingdom in the 2013-2014 season. Furthermore, the distribution of the number of cases by weeks of the year was also similar, as can be seen in Figure 3. In the 2008-2009 season there was another peak in the United Kingdom that was accompanied by an increase in the incidence of invasive disease.19,20 The rapid test for streptococcus has been available in our health care centre since 2007, and scarlet fever is documented by means of an ICD 9 code (034.1). When we reviewed the electronic medical records of our patients from that year on, we also observed an increase in cases during the 2008-2009 season (Figure 5).19,20,21 In the United Kingdom, there continued to be an incidence greater than expected in the 2014-2015 season, breaking the cyclical pattern.22
The universe of our sample consisted of the 2852 patients that comprise the two caseloads of our health care centre and that were enrolled in various schools in the town, and since we found cases in every school and nursery we believe that this was not an isolated outbreak. The total population of children in the town is of 12 479, so scarlet fever probably affected many more children. Furthermore, considering the similarities with the United Kingdom, we think it likely that the increased incidence was not restricted to our town.
Significant increases in incidence have also been seen in Chinese populations, such as Hong Kong, Shanghai, Beijing and Taiwan, although in different seasons, a difference that may have to do with these countries being geographically distant and having a different climate.23,24,25,26 However, in Poland, a country that is geographically closer to Spain and where reporting of cases is mandated, there was an increased incidence in the 2011-2012 season, when the incidence was low both in the United Kingdom and in our population.27
Given the possibility of a future increase in the incidence of invasive disease by streptococcus, we think that epidemiologic surveillance by means of mandated reporting is necessary. The United Kingdom, where this disease is the cause of great concern, is working on the development of evidence-based guidelines for the management of outbreaks in schools, nurseries and other services for children.28
The male predominance found in our study is only consistent with the data of a few other works, and there is no conclusive evidence regarding sex.1,8,9,10In our sample, diagnosis occurred at an earlier age than that reported in historical texts, and this is consistent with most recent studies, a fact that could be due to earlier enrolment of children in educational facilities. 1,8,9,10.
In our opinion, the most interesting finding in our study was the divergence of the clinical manifestations from those described in historical sources. For a disease that is considered essentially febrile, there was a high percentage of cases that presented without fever (15.4%). This could be due to the possibility of scarlet fever manifesting without fever, or to the onset of fever occurring after the development of the exanthema.
Nearly 40% of patients presented with cold symptoms, which could be interpreted as patients having a concurrent viral infection or as scarlet fever being the cause, which we consider less likely. Other authors have reported similar findings. To elucidate this issue, it would be interesting to conduct studies that include tests for the detection of respiratory virus.8
The presence of pharyngeal hyperaemia was documented in almost every case, but pharyngeal exudate was recorded in very few patients. Pharyngeal exudate is considered a typical finding in the classic literature, and it is included in the Centor and McIsaac criteria as a feature that adds to the likelihood of a streptococcal aetiology versus a viral one. This is not consistent with the low frequency in our sample or the data of other clinical studies, which calls into question the validity of these assessment scores.6,8,29,30
The most interesting finding of our study was the variability in the exanthemata. On one hand, many of the exanthemata that met the typical criteria were mild and of small extent, which make us wonder whether they may go unnoticed by caregivers as well as health care providers.
We found atypical exanthemata in 20% of the cases. All of them had been confirmed by microbiological testing, which, along with the compatible findings of the physical examination and the epidemiological context, led to the diagnosis of scarlet fever. On the other hand, it is possible that these were cases of viral infection in patients that were carriers of GAS, but we think this is not likely since all the other findings supported the scarlet fever diagnosis.
Of all cases with atypical exanthema, we would like to highlight those that presented with erythroderma, hives, and swelling in the face and the distal region of the extremities. One of the patients with generalised erythroderma and smooth skin had a prior episode with the same type of rash that resulted in admission to the hospital for suspected toxic shock; the outcome of the episode, as well as the development months later of the same exanthema with a positive streptococcus test and the resolution of symptoms after 24 hours of treatment with amoxicillin suggests that the disease that resulted in the previous hospital admission had been scarlet fever. Another atypical exanthema worth noting presented with hives compatible with urticaria. This rash developed in two febrile episodes in a single patient and was accompanied by an intensely hyperaemic pharynx and a positive rapid test for GAS, and in one of the episodes the rash also had a sandpaper feel.
Serving as a reminder that GAS can produce other diseases, in one patient scarlet fever occurred concurrently with perianal streptococcal disease. In fact, the reason for seeking care was constipation accompanied by intense pain during bowel movements. Both the pharyngeal and the perianal exudate samples tested positive for streptococcus.
Other clinical findings, such as abnormalities in the tongue, cheilitis and desquamation are not well described in our study due to its retrospective nature and the lack of a longitudinal followup.
Due to its variable clinical presentation, scarlet fever may be confused with other diseases, such as viral infections, Kawasaki disease or toxic shock. It is in these uncertain cases that microbiological testing becomes important in guiding the diagnosis. While it is true that a positive test would not suffice to rule out diseases like those we have just mentioned, combined with a quick response to antibiotic treatment it could prevent the use of more aggressive therapies and lengthy hospitalizations associated with the suspicion of more severe diseases.
When it comes to the recurrence of scarlet fever, there are no data regarding its incidence. Historical texts mention the possibility of a patient having up to three episodes, and yet we had a patient that experienced four. Recent studies are finding new pyrogenic toxin variants that make it possible for patients to suffer multiple episodes. Up to nine different toxins have already been described.5,6,11,12,14,25,31
Despite the limitations in our study, this article highlights several important clinical findings that have been reported infrequently in previous works. We need broader studies on the signs and symptoms of scarlet fever, as increased knowledge on this subject could prevent the confusion of this disease with others. We recommend performance of microbiological testing in cases in which the diagnosis is not clear. It would also be interesting to gain accurate knowledge of its epidemiology in Spain, which would require the mandated reporting of scarlet fever cases.
REFERENCES
1. Gerbe MA. Estreptococo grupo A. En: Kliegman RM, Stanton BF, Schor NF, Geme JW, Behrman RE. Nelson Tratado de Pediatría. 19.ª edición. Barcelona: Elsevier España. 2013; 955-60. [ Links ]
2. Yang SG, Dong HJ, Li FR, Xie SY, Cao HC, Xia SC, et al . Report and analysis of a scarlet fever outbreak among adults through food-borne transmission in China. J Infect. 2007;55:419-24. [ Links ]
3. Eisenhut M. Food as source of outbreaks of group A streptococcal disease. Arch Dis Child. 2011;96:323. [ Links ]
4. Schwartz RH, Kim D, Martin M, Pichichero ME. A Reappraisal of the minimum duration of antibiotic treatment before approval of return to school for children with Streptococcal pharyngitis. Pediatric Infect Dis J. 2015;34:1302-4. [ Links ]
5. Herranz Jordán B, Acitores Suz E, Payá López A, Hernández Merino A, Lamela Lence MT, Sánchez Casado M, et al . Escarlatina recurrente: presentación de cuatro casos. Rev Pediatr Aten Primaria. 2001;3:551-60. [ Links ]
6. Chiesa C, Pacifico L, Nanni F, Orefici G. Recurrent attacks of scarlet fever. Arch Pediatr Adolesc Med. 1994;148:656-9. [ Links ]
7. Pericas Bosch J. Escarlatina. FMC. Form Med Contin Aten Prim. 2002;9:352-3. [ Links ]
8. Ortigosa Gómez S, Sánchez Buenavida A, Crehuet Almirall M, Martínez-Roig A. Diagnóstico de escarlatina en 151 casos en el servicio de urgencias pediátricas durante 2006-2008. Rev Enferm Infect Pediatr. 2011;24:154-61. [ Links ]
9. Casaní Martínez C, Morales Suárez-Varela M, Santos Durántez M, Otero Reigada MC, Pérez Tamarit D, Asensi Botet F. Estudio epidemiológico de un brote de escarlatina. Rev Pediatr Aten Primaria. 2001;3:41-9. [ Links ]
10. Fernández-Prada M, Martínez-Diz S, Colina López A, Almagro Nievas D, Martínez Romero B, Huertas Martínez J. Brote de escarlatina en un colegio público de infantil y primaria en Granada en 2012. An Pediatr (Barc). 2014;80:249-53. [ Links ]
11. Silva-Costa C, Carriço JA, Ramírez M, Melo-Cristino J. Scarlet fever is caused by a limited number of Streptococcus pyogenes lineages and is associated with the exotoxin genes ssa, speA and speC. Pediatr Infect Dis J. 2014;33:306-10. [ Links ]
12. Schmitz FJ, Beyer A, Charpentier E, Henriques Normark B, Schade M, Fluit AC, et al . Toxin-gene profile heterogeneity among endemic invasive european group a Streptococcal isolates. J Infect Dis. 2003;188:1578-86. [ Links ]
13. Po-Chuang W, Wen-Tsung L, Shyi-Jou C, Chih-Chien W. Molecular characterization of group A streptococcal isolates causing scarlet fever and pharyngitis among young children: a retrospective study from a northern Taiwan medical center. J Microbiol Immunol Infect. 2013;47:304-10. [ Links ]
14. Sanz JC, Bascones MA, Martin F, Sáez-Nieto JA. Escarlatina recurrente por reinfección reciente causadas por cepas no relacionadas de Streptococcus pyogenes. Enferm Infecc Microbiol Clin. 2005;23:388-92. [ Links ]
15. Ficha técnica. Test rápido OSOM(r) Strep A Test Genzyme. En: Laboratorios LETI Diagnósticos [en línea] [consultado el 08/09/2016). Disponible en http://diagnosticos.leti.com/es/osom-strep-a-test_7735 [ Links ]
16. Llor C, Hernández Anadón S, Gómez Bertomeu FF, Santamaría Puig JM, Calviño Domínguez O, Fernández Pages Y. Validación de una técnica antigénica rápida en el diagnóstico de la faringitis por estreptococo betahemolítico del grupo A. Aten Primaria. 2008;40:489-96. [ Links ]
17. Gieseker KE, McKenzie T, Roe MH, Toss JK. Comparison of two rapid Streptococcus pyogenes diagnostic tests with a rigorous culture standard. Pediatr Infect Dis J. 2002;21:922-7. [ Links ]
18. Wright CM, Williams G, Ludeman L. Comparison of two tests for detecting group A streptococcal pharyngitis in the pediatric population at Wright-Patterson air Force Base. Mil Med. 2007;172:644-6. [ Links ]
19. Guy R, Williams C, Irvine N, Reynolds A, Coelho J, Saliba V, et al . Increase in scarlet fever notifications in the United Kingdom, 2013/2014. Euro Surveill. 2014;19:20749. [ Links ]
20. Lamden KH. An outbreak of scarlet fever in a primary school. Arch Dis Child. 2011;96:394-7. [ Links ]
21. Group A streptococcal infections: third update on seasonal activity, 2008/09. Health Protection Report. En: Health Protection Agency (HPA) [en línea] [consultado el 08/09/2016]. Disponible en http://www.hpa.org.uk/hpr/archives/2009/news1309.htm#igas3 [ Links ]
22. Group A streptococcal infections: sixth update on seasonal activity, 2014/15. En: Public Health England Health [en línea] [consultado el 08/09/2016]. Disponible en http://www.gov.uk/government/uploads/system/uploads/attachment_data/file/442058/hpr2315_sf-gas6.pdf [ Links ]
23. Luk EYY, Lo JYC, Li AZL, Lau MCK, Cheung TKM, Wong AYM, et al . Scarlet fever epidemic, Hong Kong, 2011. Emerg Infect Dis. 2012;18:1658-61. [ Links ]
24. Chen M, Yao W, Wang X, Li Y, Chen M, Wang G, et al . Outbreak of scarlet fever associated with emm12 type group A Streptococcus in 2011 in Shanghai, China. Pediatr Infect Dis J. 2012;31:158-62. [ Links ]
25. Wu PC, Lo WT, Chen SJ, Wang CC. Molecular characterization of Group A streptococcal isolates causing scarlet fever and pharyngitis among young children: A retrospective study from a northern Taiwan medical center. J Microbiol Immunol Infect. 2014;47:304-10. [ Links ]
26. Yang P, Peng X, Zhang D, Wu S, Liu Y, Cui S, et al . Characteristics of group A Streptococcus strains circulating during scarlet fever epidemic, Beijing, China, 2011. Emerg Infect Dis. 2013;19:909-15. [ Links ]
27. Staszewska E, Kondej B, Czarkowski MP. Scarlet fever in Poland in 2012. Przegl Epidemiol. 2014;68:209-12. [ Links ]
28. Public Health England. Interim guidelines for the public health management of scarlet fever outbreaks in schools, nurseries and other childcare settings. En: Public Health England [en línea] [consultado el 08/09/2016]. Disponible en http://www.gov.uk/government/publications/scarlet-fever-managing-outbreaks-in-schools-and-nurseries [ Links ]
29. Roggen I, van Berlaer G, Gordts F. Centor criteria in children in a paediatric emergency department: for what it is worth. BMJ Open. 2013;3:e002712. [ Links ]
30. Cohen JF, Cohen R, Levy C, Thollot F, Benani M, Bidet P, et al . Selective testing strategies for diagnosing group A streptococcal infection in children with pharyngitis: a systematic review and prospective multicentre external validation study. CMAJ. 2015;187:23-32. [ Links ]
31. Casaní Martínez C, Morales Suárez-Varela M, Santos Durántez M, Otero MC, Pérez Tamarit D, Asensi Botet F. Escarlatina recurrente. An Esp Pediatr. 1999;51:300-2. [ Links ]











 text in
text in