Introduction
Latent tuberculosis infection affects 25% of the world's population. Persons with LTBI are a reservoir of Mycobacterium tuberculosis and run a 5-10% risk of reactivating tuberculosis throughout their lives. This risk is a variable one and is considered to be higher in the first five years after the initial infection1. Eliminating TB is not a feasible proposition as long as this reservoir exists2.
The WHO assembly adopted the Global End TB strategy in 2014 to intensify efforts to eliminate TB worldwide. The aim is to reduce the incidence of TB by 90% before 20352.
There has recently been a constant reduction of incidence of TB in Spain3. Cases have been limited to sub-groups of the population with medical, social or behavioural risk factors, such as infection by HIV, homeless persons or drug users. In some areas, over 50% of cases are present in the immigrant population.
The reactivation of TB amongst infected persons can be avoided by preventive therapy, which has a rate of efficacy of 60 to 90%4. However, massive study and treatment of LTBI is not feasible, because the diagnostic tests have certain limitations, there is the risk of side effects and the costs are high. Benefits can outweigh risks for infected persons in those groups where the risk of the disease progressing is high (Table 1)4.
Table 1. Recommendations of the World Health Organisation on screening of latent tuberculosis infection in developed countries with low incidence levels of tuberculosis cases (<10 cases x 105 inhabitants).
| Group A Systematic screening required | − Persons infected with HIV. |
| − Adults and children in contact with pulmonary TB patients. | |
| − Patients starting biological therapy. | |
| − Patients in renal replacement therapy. | |
| − Persons receiving organ or blood transplants. | |
| − Patients with silicosis. | |
| Group B Consider screening based on local epidemiology and available resources | − Prison inmates. |
| − Medical personnel. | |
| − Immigrants from countries with high load of TB. | |
| − Homeless people. | |
| − Illegal drug users. | |
| Group C Systematic screening not required* | − Diabetic. |
| − Persons with dangerous alcohol consumption levels. | |
| − Smokers. | |
| − Underweight persons. |
Note. *Unless the above mentioned recommendations are applied. TB: tuberculosis; HIV: human immunodeficiency virus.
The WHO drew up a series of recommendations about who should form part of a systematic screening of LTBI, according to the risk of exposure to M. tuberculosis and of developing active TB, depending on the epidemiology and resources available (Table 1). The guidelines set out to make an impact on the reservoir of infection in countries where the incidence of TB is less than 10 cases per 100,000 inhabitants4.
The aim behind the efforts to prevent and control TB in Spain are to ensure that the incidence of TB is lower than the level of pre-elimination: 1 case per 100,000 inhabitants by 20354,5. To achieve this objective, control of LTBI needs to be included in strategies to prevent and control TB, as recommended by the European Centre for Disease Prevention and Control (ECDC) to the member states of the economic area of the European Union/European Economic Area (EA/EEA)6.
The aim of this review is to present the strategies for groups at risk that are candidates for systematic LTBI detection and therapy.
2. Screening of latent tuberculosis infection in high risk groups
LTBI screening is only justified if the possible detection of an infection will lead to treatment to prevent TB. Two categories of high risk groups can be defined: persons with a higher risk of LTBI, but who do not run a higher risk of progressing to active tuberculosis, and persons with LTBI who do run a higher risk of a progression to active TB in comparison to other persons with LTBI. Some specific high risk groups, such as persons infected with HIV, patients with chronic kidney disease, contacts of cases of pulmonary TB, the homeless and immigrants, may be included in one or both categories. There is sufficient evidence of the profitability of screening at individual and community levels in the groups4-6 described below.
2.1. Immunosuppressed persons
2.1.1. Persons infected by HIV (adults, adolescents and children)
Infection with HIV increases the risk of TB, and at the same time, TB increases viral replication and imposes a higher risk of progression of the disease. It has been amply demonstrated that therapy for LTBI is highly effective in preventing TB amongst persons infected by HIV7.
Screened population: all HIV-positive patients (adults, adolescents and children).
Screening method: any of the tests currently available: tuberculin skin test or IGRA. Using both tests is not recommended. The IGRA should only be used if the patient has <200 CD4 and the result is negative when using the tuberculin test.
Place of screening: specialist clinical units (HIV or TB) that treat this type of patient. Alternatives include other areas where these patients are temporarily housed, such as prisons and drug treatment centres.
2.1.2. Persons starting biological therapies
Systematic LTBI research and treatment has proven to be effective in reducing TB associated with immunotherapies8. The published results mention treatment with isoniazid, although short regimens with rifampicin, with or without isoniazid, are also widely used.
Screened population: any person who needs biological therapy. Therapy is recommended for all patients regardless of the treatment due to the fact that they receive different immunosuppressive treatments sequentially. In those cases where LTBI is not detected, the tests should only be repeated if there is exposure to risk over the course of the treatment.
Screening method: possible with either of the tests available (TST and IGRA). Testing with both is not recommended.
Place of screening: specialist clinics (TB units or similar), where the clinical, radiological and epidemiological data is evaluated and used to decide on the best LTBI therapy.
2.1.3. Dialysis patients
Persons with advanced chronic kidney disease are a group at risk of TB. Most of the patients are already indicated for testing as they are candidates for transplant, which is the first choice therapy for advanced kidney disease9.
Screened population: all persons in renal replacement therapy.
Screening method: can be done with either test (TST and IGRA).
Place of screening: specialist clinics (TB units or similar), where the clinical, radiological and epidemiological data is evaluated and used to decide on the best LTBI therapy.
2.1.4. Persons obliged to undergo an organ transplant
The incidence of TB amongst organ recipients is higher than amongst the general public. The risk depends on the type of transplant (highest in lung transplants and lowest in transplant of hematopoietic progenitors) and the prevalence of tuberculosis infection in the population10.
Screened population: patients obliged to undergo an organ transplant.
Screening method: can be done with either test (TST and IGRA).
Place of screening: specialist clinics (TB units or similar), where the clinical, radiological and epidemiological data is evaluated and used to decide on the best preventive therapy.
2.2. Recent immigrants
Immigrants have a high risk of TB because of potential reactivation of LTBI acquired in their country of origin, frequent trips to areas of high incidence and transmission in communities of immigrants in recipient countries11,12. Immigrants present one fourth of the cases of TB in the EU/EEA. In many areas of Spain, 50% of new cases are also presented amongst immigrants.
Screened population: all immigrants under 35 years of age, with two years or less of residence, from countries with a high incidence of the disease (incidence >100 cases per 100,000 inhabitants).
Screening method: the recommended test is the TST. In cases vaccinated with the bacillus Calmette-Guérin (BCG) with a positive TST, the option of using the IGRA may be considered to confirm or rule out an LTBI.
Place of screening: this should be carried out in primary care, with an opportunistic strategy so as to standardise detection. More active screening strategies linked to obtaining a health card or in paediatric consultations for parents of recently arrive children can also be considered.
2.3. Prison inmates
TB screening when an inmate enters prison is habitually practiced and prisons can comply with the objectives of the WHO and the ECDC for countries with low incidence. It is estimated that the prevalence of LTBI amongst inmates is 40-50% and it is believed that the global ratio is two or three times greater than the estimated prevalence for the Spanish population13.
Screened population: all prison inmates who have not been previously diagnosed with LTBI or TB.
Screening method: the recommended test is the TST. The use of IGRA may be considered for persons vaccinated with BCG with a positive TST so as to confirm or rule out an LTBI.
Place of screening: the prison medical services during initial examination, carried out in the first 24 hours after entering prison.
2.4. Intravenous drug users and homeless persons
According to the WHO, the detection of LTBI amongst intravenous drug users and homeless persons should be restricted to countries with low incidence, and take into consideration local resources and epidemiology. In a study carried out at the Drassanes-Vall d'Hebron centre, the prevalence of TB amongst users of hostels and soup kitchens was 3 persons per 1,000, and 6 per 1,000 amongst drug users, although the patients sometimes belonged to more than one high risk group14. There are figures in other studies that show an excess incidence that is 17 times greater than amongst the general population15.
The profile of the intravenous drug users who arrived at harm reduction programmes (HRP) had a combination of characteristics of vulnerability to TB: 27% of the persons were infected by HIV, 49% were foreigners and 43% were homeless. The figures for intravenous drug users attending drug dependency care and monitoring centres (CMC) showed that 25% were HIV positive, 16.2% were immigrants and 13.3% were homeless.
2.4.1. Intravenous drug users
Common protocols should be used to rule out tuberculosis in persons who go to HRPs or CMCs. The option of including persons who are infected at the end of the screening in LTBI treatment shall be considered: in cases of HIV infection or another immunosuppressive disease, in persons under 35 years of age and persons who present a recent conversion6,7.
Screening method: either test (TST or IGRA), but using both is not recommended. If the TST is used, the IGRA test should also be considered if there is immunosuppression (CD4 <200) and the patient gives a negative result.
Place of screening: the CMC or HRP unit in line with common protocols.
2.4.2. Homeless persons
Studies should be restricted to persons with a record of homelessness of at least one year. The option of initiating LTBI treatment shall be considered for people who are found to have LTBI at the end of screening: in cases of HIV infection or another immunosuppressive disease, in persons under 35 years of age and persons who present a recent conversion6,7.
Screening method: either test (TST or IGRA), but using both is not recommended. If the TST is used, the IGRA test should also be considered if there is immunosuppression (CD4 <200) and the patient gives a negative result.
Place of screening: the public health services, managers of centres and other resources, soup kitchens and care centre where clinical monitoring takes place should all coordinate to ensure supervision of the LTBI treatment.
2.5. Workers in high risk environments: medical and social welfare centres, persons and hostels
Medical personnel who work with TB patients are exposed to TB and run a higher risk of catching it than the general public. A study published in our centre showed a prevalence of 14.6%16. A study published in Montreal on non-medical personnel working in high-risk hostels and prisons showed that prevalence was similar to levels in medical staff17.
Professionals should be classified according to the risk of LTBI in: high risk activities or work (cough provocation tests, bronchoscopy, micro-bacteria labs, staff carrying out autopsies on tuberculosis cases); intermediate risk activities (staff who come into direct and regular contact with patients, hostel workers); low risk activities (workers in health centres with minimal contact with patients, workers in contact with patients or inmates with very occasional cases of TB).
Screening method: the recommended test is TST (≥10 mm). Cases with BCG vaccination and positive tuberculin tests should have receive the IGRA to confirm or rule out infection. It has been shown that yearly screening of medical personnel is not feasible. It is therefore proposed to carry out a baseline assessment and only carry out a yearly or biannual test on intermediate or high risk workers with a negative baseline test (in settings where there may be patients with tuberculosis). The TST and/or IGRA should be carried out to rule out LTBI and it should be compared with previous tests.
Place of screening: the occupational health and safety units carry out the initial health evaluation when the activity commences or after a task with new health risks is assigned. The evaluation should include LTBI detection. If a worker comes into contact with an infected patient without isolation procedures, a contacts study should be set in motion. The TST and/or IGRA tests should be repeated in line with established guidelines for yearly or biannual health assessments when working in certain services with a risk of tuberculosis infection.
3. Diagnostic tests for latent tuberculosis infection
LTBI is a clinical diagnosis that shows a previous infection by M. tuberculosis, and excludes active TB. The tests available for diagnosing tuberculosis infection include the TST and the IGRA5,6.
3.1. The tuberculin test (Mantoux test)
The tuberculin reaction consists of putting a person in contact with an extract of TB bacillus (PPD or purified protein derivative), in order to detect their sensitivity to M. tuberculosis. Tuberculin is an extract taken from a TB bacillus culture. The one used in Spain is PPD-RT 23, with a dose of two units (0.1 mL). The Mantoux method is used where the tuberculin is administered intradermally in the mid-third of the forearm. The reading should takes place 48-72 hours later, using the Sokal method. The test may present false negatives and positives and should be interpreted according to the risk groups (Table 2).
Table 2. Interpretation of positive result of tuberculin test.
| PT ≥5 mm considered positive |
| Patients infected with HIV. |
| Close contact with persons who have pulmonary or laryngeal TB. |
| Children <5 years not previously vaccinated with BCG and who come from countries with a high incidence. |
| Radiological evidence of previously cured TB, in patients who were did not receive therapy of any recognised efficacy. |
| Persons receiving organ transplants. |
| persons with depressed immune systems for other reasons: e.g. receiving doses equivalent to ≥15 mg/day of prednisone for a month or more, or persons starting biological therapy. |
| Persons with advanced kidney disease. |
| PT ≥10 mm considered positive |
| Immigrants from areas with high prevalence of TB in last five years. |
| Intravenous drug users. |
| Residents and employees in places where high risk situations take place: medical facilities with exposure to TB, microbiology lab staff, prisons, hostels, refuges for the homeless, geriatric residences, drug dependency centres, volunteers and military personnel in areas with high levels of endemic tuberculosis. |
| Persons with risk factors for TB different from HIV infection. |
| Patients with clinical conditions such as: silicosis, diabetes, chronic kidney failure undergoing renal replacement therapy, blood diseases (leukaemia and lymphomas), other malignant tumours (carcinoma of head, neck or lung), weight loss of over 10% under ideal weight, gastrectomy, jejunoileal bypass, celiac disease. |
| Children <5 years (except for children from countries with a high incidence and not vaccinated with BCG). |
| Children and adolescents exposed to adults who belong to groups with a high risk of progression of TB. |
| PT ≥15 mm considered positive |
| Vaccinated persons, unless they already meet one of the above criteria. |
| Persons without known risk factors and under risk of infection (administrative screening). |
Note. BCG: bacillus Calmette-Guérin; TT: tuberculin test; TB: tuberculosis; HIV: human immunodeficiency virus.
The tuberculin conversion is when an induration equal to or more than 10 mm is detected in a person with a negative response to tuberculin two years previously. In other words, a there is a recently acquired TB infection if the booster effect has been ruled out.
The booster effect can be observed in previously sensitised persons. In the first TST, such persons present a negative result because their reactivity had dissipated. This happens with people infected by M. tuberculosis, environmental mycobacteria and those vaccinated by BCG. When the TST is repeated a week later, the result is positive due to reinforcement of the immune response caused by the test itself.
3.2. Interferon gamma release assay
IGRAs are an immunological method that quantify the immune response in vitro. They are based on the production of interferon gamma (IFN-g) by T-cells of previously infected persons when they come into contact again with micro-bacterial antigens. IFN-g is an important cytokine in the control of TB infection. It activates the infected macrophages with the release of interleukin-1 and the alpha tumoral necrosis factor, which limit the growth and multiplication of the mycobacteria. These tests use more specific antigens of M. tuberculosis complex, thus avoiding cross reaction due to infection from other non-TB mycobacteria and the strain of the vaccine with BCG.
There are two methods currently on the market: ELISA, or enzyme-linked immunosorbent assay (QuantiFERON®-TB Gold Plus); and ELISpot, or enzyme-linked immunospot assay (T-SPOT®.TB)18. They are described in greater detail below.:
QuantiFERON®-TB Gold Plus: an ELISA technique that uses peripheral blood and evaluates the production of IFN- in response to the stimulation of blood lymphocytes with specific antigens of M. tuberculosis. The test is interpreted by quantifying the difference between the concentration of IFN-g produced in the sample exposed to antigens and the negative control sample. If the difference is equal to or more than 0.35 U/mL, and represents 25% or more of the value obtained in the control sample, the result is considered to be positive.
T-SPOT®TB: an ELISpot technique that uses mononuclear peripheral blood cells. The dish is incubated at 37 oC for 18-22 hours and then the immunospot is carried out to enable the number of IFN producing cells to be quantified (number of spots). Interpretation is based on the spot count in each vial. In technical terms, T SPOT® requires more blood, more preparation time and more work than QuantiFERON®.
3.3. Sensitivity and specificity of diagnostic assays
There is no benchmark for diagnosing tuberculosis infection and it is therefore difficult to establish the sensitivity and specificity of these new methods. The TST is cheap and widely used, but is not very specific in persons vaccinated with the BCG vaccine. It also has cross reations with environmental bacteria and is not very sensitive in immunodepressed persons. The specific tuberculosis antigens of the IGRA techniques (early secreted antigenic target-6 [SAT-6], cultured filtrated protein-10 [CFP-10] and TB 7.7) are absent in Mycobacterium bovis and in most non-tuberculosis mycobacteria. Therefore, the specificity of the IGRA tests for M. tuberculosis is higher than the one in the TST (Table 3).
Table 3. Advantages and disadvantages of IGRA tests compared to tuberculin tests.
| Advantages of an in vitro diagnosis in comparison to TT |
| Eliminates subjectivity. |
| Absence of false positives in persons vaccinated with the BCG vaccine or in persons infected by atypical mycobacteria. |
| Control of false negatives in patients infected by HIV. |
| Avoids treatment of latent tuberculosis infection. |
| Disadvantages of an in vitro diagnosis in comparison to TT |
| More expensive. |
| Difficult to use in community screenings (issues with blood extraction, transport and conservation). |
Note. BCG: bacillus Calmette-Guérin; IGRA: interferon gamma release assay; TT: tuberculin test; HIV: human immunodeficiency virus.
The capacity of the TST and IGRA to predict the development of TB is limited, since a large number of individuals with positive TST or IGRA results would not progress to full tuberculosis6. Despite this limitation, IGRA tests have improved LTBI diagnosis, because its higher specificity has enabled the number of unnecessary preventive treatments to be reduced. The IGRA test have also improved detection of LTBI in immunodepressed patients.
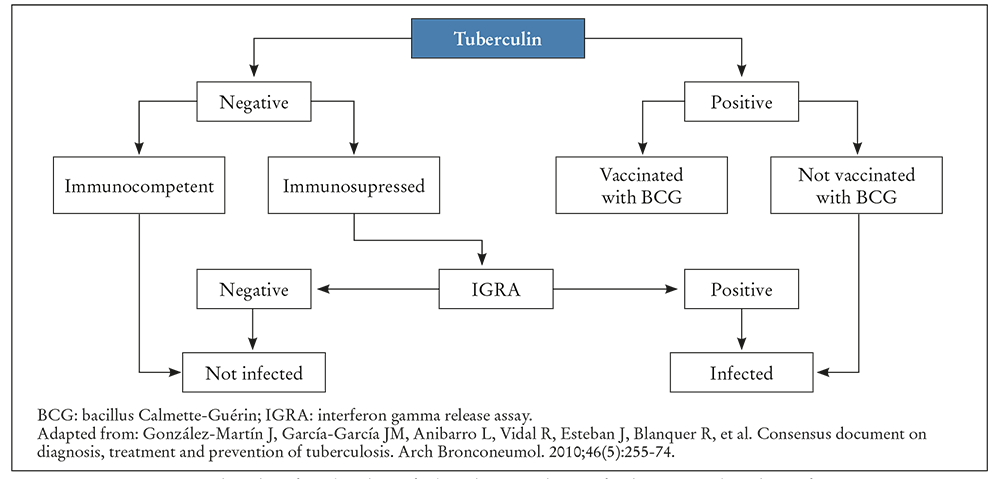
The mismatch between the TST and IGRA in HIV positive or immunodepressed patients and those vaccinated with BCG indicates that IGRA tests should be available for use in such situations. There are studies that suggest that these tests could be more cost effective in the medium and long term than TST18. Figure 1 shows the algorithm of combined use of TST and IGRA for diagnosing tuberculosis infection presented in the Consensus Document on the Diagnosis and Treatment of Tuberculosis by the Spanish Association of Respiratory Diseases (SEPAR). Choosing one or the other technique depends on a number of factors that go beyond their validity, and which can be regarded as comparable. There are other logistical factors to take into consideration, such as avoiding venipuncture (TST) or patients not requiring a second visit to obtain the IGRA results.
4. Treatment of latent tuberculosis infection
Any person who runs a high risk of progression to full blown tuberculosis (Table 1, group A), and who has a positive result in the LTBI detection tests should be treated for latent tuberculosis infection if he meets the following criteria: he has no symptoms, signs or radiological evidence of active tuberculosis, is willing to comply with the complete treatment regime and has no medical contraindications.
If there is evidence of previous tuberculosis treatment, then further therapy is not necessary except in exceptional cases such as persons at risk of exposure to patients with tuberculosis bacilli who meet any of the following conditions: HIV positive, undergoing a medical process where there is a high risk of developing active tuberculosis, children who have undergone an intense exposure to secondary cases or conversions. The persons included for LTBI screening and therapy in Table 1 should be subject to the same criteria.
5. Therapeutic options for treating latent tuberculosis infection
Latent tuberculosis infection is mainly treated with isoniazid and rifampicin, either separately or in combination. Isoniazid in regimens of 6-9 months is the reference drug for treating LTBI5,6. However, the treatment's duration and toxicity (especially to the liver) act as serious obstacles to its use. As a result, new shorter treatment regimens of 3-4 months have been introduced, using a range of anti-tuberculosis drugs such as rifampicin and isoniazid or rifampicin alone5,6.
All infected patients should be monitored to detect any possible side effects. The regimens that present best compliance are short ones of 3-4 months, with rifampicin plus isoniazid (3RH) or rifampicin alone (4R), respectively (Tables 4 and 5). The regimens with rifampicin have been shown to be efficacious when compared to isoniazid in reducing the risk of tuberculosis and also present reduced liver toxicity and better adherence than isoniazid (82% compared to 69%)19.
Table 4. Therapeutic options for treating latent tuberculosis infection.
| Drugs | Duration | Interval | HIV classification (-) | HIV classification (+) |
|---|---|---|---|---|
| Isoniazid | 9 months | Daily | A (II) | A (II) |
| Isoniazid | 6 months | Daily | B (I) | C (I) |
| Rifampicin + Isoniazid | 3 months | Daily | A (II) | A (II) |
| Rifampicin | 4 months | Daily | B (II) | B (III) |
Note. Weight of recommendation: A: preferable; B: acceptable alternative; C: only use when A and B are not possible. Quality of evidence: I: evidence from random clinical assays; II: evidence from non-random clinical assays. III: expert opinion. HIV: human immunodeficiency virus.
Table 5. Recommended dose of drugs for treating latent tuberculosis infection.
| Therapeutic regimen | Dose for adults and children ≥12 years | Dose for children <12 years | Maximum dose |
|---|---|---|---|
| Isoniazid only, daily for 6 or 9 months | 5 mg/kg/day | 10 mg/kg/ day (range, 7-15 mg) | 300 mg |
| Rifampicina only, daily for 4 months | 10 mg/kg/day | 15 mg/kg/ day (range, 10-20 mg) | 600 mg |
| Isoniazid and rifampicin, daily for 3 months | Isoniazid: 5 mg/kg/ day Rifampicin: 10 mg/kg/day 15 mg (range, 10-20 mg) | Isoniazid: 10 mg/kg/ day (range, 7-15 mg) Rifampicin: 15 mg/kg/ day (range, 10-20 mg) | Isoniazid, 300 mg Rifampicin, 600 mg |
In practical terms, the therapeutic regimen should be selected with the following points in mind: the coexistence of an underlying disease, possible drug interactions and the fact that the most appropriate regimen should be agreed with the patient to ensure compliance. In fact, the recommended regimen for ensuring compliance is a short regimen (3RH or 4R) if there are no contraindications. Isoniazid (6H-9H) is recommended for the following cases: intolerance to or incompatibility with rifampicin, patient resistance to rifampicin, and pregnancy or breastfeeding. Rifampicin (4R) is recommended for the following: intolerance to or incompatibility with isoniazid and resistance of the index case to isoniazid19.
Ensuring adherence is absolutely essential in LTBI treatment. Defective adherence is associated with factors such as lack of family support, alcoholism, immigration and social issues. There is no consensus on which particular method can be used to improve adherence, but health education and monitoring tolerance throughout treatment are essential backups in ensuring that the therapy continues. Testing for hepatic enzymes before therapy and one month afterwards is recommended. Patients with underlying diseases, such as transplant candidates, may require more stringent monitoring. Children may be exception in this case as hepatic toxicity is rare, and therapy can commence with no prior analyses and is only necessary if any symptoms are detected19.
6. Conclusions
The epidemiological data indicates a need to deal with LTBI if we want to make any progress in completely eliminating TB. Given the estimates of high prevalence of LTBI and the limited number of tests and therapies that are currently being carried out, new efforts need to be made to reduce the number of persons with LTBI in the community. Such efforts should include a recording and monitoring system to supervise progress in populations and patients, an increase in testing to detect LTBI in high risk groups, more short regimens of LTBI treatment and the active participation of communities and health services20. Including new tools that offer better indications of the risk of reactivation in patients, along with new and shorter LTBI treatment regimens, may improve TB elimination programmes.