Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Enfermería Global
versión On-line ISSN 1695-6141
Enferm. glob. vol.18 no.55 Murcia jul. 2019 Epub 21-Oct-2019
https://dx.doi.org/10.6018/eglobal.18.3.347541
Originals
Influence of cardiovascular risk factors on the occurrence of foot risk, prior to the complementary study with infrared thermography
1PhD. Department of Nursing and Physiotherapy, Universidad de Castilla-La Mancha, Talavera de la Reina, Toledo. Spain. alvaro.astasio@gmail.com
2PhD. Department of Nursing, University of Extremadura, Plasencia, Cáceres. Spain.
Introduction
Diabetes Mellitus is a public health problem. Diabetic foot is a degeneration of the vascular structure of the feet, whose patients present neurological problems, necessary to identify in the shortest possible time.
Objective
The objective of the study is to analyze the influence of risk factors on the appearance of risk foot, as complementary data to the study by infrared thermography.
Method
A descriptive, cross-sectional and observational study was proposed on a sample of 479 subjects framed in two groups, group cases (people with diabetes) and control group (people without diabetes). The group cases comprised a total of 277 people, with an average age of 63.41 years, [138 men (49.8%) and 139 women (50.2%)]. In the same way for the control group, the number consisted of 202 users, with an average age of 61.92 years, [99 men (49%) and 103 women (51%)]. The taking of images has been carried out with the FLIR E60bx® camera (FLIR® Company, Boston, USA). The statistical analysis of the data obtained was carried out using the IBM SPSS Statistics 22 statistical package.
Conclusion
It can be concluded by stating that the study of the different risk factors is key in the diagnosis of risk foot. It can be clearly established that age is an obvious condition, since advanced ages correspond to a BMI and greater abdominal perimeter. Together, the analysis by infrared thermography in the assessment of the foot risk is useful for the diagnosis and prevention of compromised areas of the foot, thus avoiding the obvious trigger in the damages of a diabetic foot.
Key words: Risk foot; Diabetic foot; Infrared thermography; Medical thermography; Diabetes
INTRODUCTION
According to the World Health Organization (WHO), the global prevalence of diabetes mellitus (DM) in 2014 was 9% among adults older than 181.
Diabetic foot (DF) ulcers are one of the main complications of these patients' feet. They can be prevenTable with an appropriate strategy that includes screening, risk classification, and effective prevention and treatment measures2.
The risk of ulcers or amputations is raised in diabetics who have the following risk factors3:
Old age or more than 10 years evolution of their DM.
Poor or no glycæmic control (high HbA1c).
A loss of sensation in the distal region of the foot in response to a 10-g Semmes-Weinstein monofilament examination.
Peripheral neuropathy with numbness and loss of protective sensation (LOPS).
Smoking.
Dyslipidæmia and arterial hypertension.
Deformities or hyperkeratosis of the foot.
Heloma or pre-ulcerative wound.
Peripheral vascular disease (PVD).
History of ulceration or amputation in the foot.
Amputation.
Inadequate footwear.
Visual deterioration (advanced retinopathy).
Reduced joint mobility.
Diabetic nephropathy or renal failure (especially dialysis patients).
Low socioeconomic level, alcoholism, social isolation, or poor foot hygiene.
There thus exist predisposing factors such as diabetic neuropathy associated with macro- and micro-angiopathy that give rise to a vulnerable foot of high risk, triggering factors such as mechanical trauma leading to an ulcer or necrosis, and aggravating factors which will determine the prognosis of the extremity and include infection that causes extensive tissue damage, ischæmia that delays healing, and neuropathy that prevents identification of the lesion and the precipitating factor4.
Infrared thermography (IRT) is a technique which, by capturing the radiation in the infrared of the electromagnetic spectrum, allows the temperature or the radiated heat to be measured at a distance from a body, without needing physical contact with that body5. It is a safe, non-invasive, and low-cost technique that allows rapid registration of the energy radiated from the body without contact with the patient6.
Blood flow is the main mechanism of heat transfer in the human body. The said heat surrounds the blood flowing in arteriovenous paths, and emanates from the surface of the skin7. Temperature gradients in regions affected with vascular disorders are shown up by IRT, indicating abnormal blood flow. The temperature contrast in affected regions is approximately 0.7 to 1.0°C above normal regions due to slow circulation of the blood8. Some publications have also suggested that variations in skin temperature of 2.2°C could be useful in monitoring the skin9.
To obtain more precise knowledge of the appropriate tests to carry out, health professionals should review the standard recommendations for complete foot examinations10.
MATERIAL AND METHODS
A descriptive, cross-sectional, and observational study was proposed11. The sample consisted of 479 subjects corresponding to two groups - cases (subjects with diabetes) and control (subjects without diabetes). The cases group comprised 277 subjects, mean age 63.41 years, 138 men (49.8%) and 139 women (50.2%). The control group comprised 202 subjects, mean age 61.92 years, 99 men (49%) and 103 women (51%).
The case group included all patients with an accurate diagnosis of diabetes mellitus according to the criteria for diagnosis of diabetes of the American Diabetes Association (ADA)10. The inclusion criteria were: to be diagnosed with diabetes mellitus, and to accept participation by signing the appropriate informed consent. The exclusion criteria were: fracture or recent surgery involving the lower limb, sensory-perceptive limitations that would prevent response to the evaluation or could interfere in the follow-up, gestational diabetes, and/or plantar hyperhidrosis.
The control group comprised subjects without diagnosed diabetes mellitus, residents of the Autonomous Community of Extremadura, of both sexes, who agreed to participate in the study by signing the appropriate informed consent. The exclusion criteria were: fracture or recent surgery involving the lower limb, sensory-perceptive limitations that would prevent response to the evaluation or could interfere in the follow-up, not agreeing to participate in the study, and/or plantar hyperhidrosis.
The data collection was carried out in the facilities of the Podiatric Clinic of the University of Extremadura in Plasencia (Cáceres, Spain) and in various health centres belonging to the Extremadura Health Service of the Regional Department (Consejería) of Health and Social Policies. The mean temperature in the room the tests were carried out in was 22.63 ± 2.28 °C, and the relative humidity was 33.50 ± 8.10 %12.
The diagnosis of diabetes was confirmed by evaluating the levels of glucose and glycosylated hæmoglobin in the last blood test13. An interview was conducted with all the subjects of the study, and a routine physical examination was carried out. To minimize inter- and intra-explorer bias, all interventions were carried out by two qualified and trained clinicians14.
The variables used were grouped into seven sections: anthropometric data (age, weight, height, BMI, and waist circumference); vital signs (body temperature, systolic blood pressure, diastolic blood pressure, pulse, and oxygen saturation); diagnosis and evolution of DM (years of diagnosis, basal glycæmia, HbA1c, total cholesterol, and HDL and LDL cholesterol); risk factors (arteriosclerosis, hypertension, ischæmic heart disease, obesity, and consumption of tobacco and alcohol); physical activity related (sedentary lifestyle, walking, and running or similar); neuropathy related (Semmes-Weinstein monofilament, 128 Hz tuning fork, and patellar and Achilles reflexes); and vasculopathy related (popliteal, posterior tibial, and pedal pulses, inspection of the skin, and assessments of capillary refill, pain when walking, and pain at rest).
The researchers informed the subjects of the study about its origin and purpose, use of the data, and, in general, about all aspects that might influence their participation. The investigation was based on effective fulfilment of the principles of ethics, including the signing of informed consent prior to conducting the study, and taking into account all aspects established in this regard and the favourable report of the Bioethics and Biosecurity Commission of the University of Extremadura, following the principles established in the Helsinki Declaration of the World Medical Association.
Statistical analyses were performed using the IBM SPSS Statistics 22 statistical package. For the descriptive analysis, the number of patients (N), mean value, standard deviation (SD), minimum, maximum, and 25th, 50th, and 75th percentiles were calculated. A t-test for independent samples was applied to compare independent samples when the values of the variables met the criteria for parametric statistics. All decisions were made at a 95% confidence level.
RESULTS
In comparing the anthropometric data and vital sign parameters of the diabetes group with those of the control group, we found the following significant differences (p<0.050): greater weight (p=0.048), BMI (p=0.042), waist circumference (p<0.001), systolic blood pressure (p=0.003), diastolic blood pressure (p=0.015), and pulse (p<0.001) in the diabetic group than in the control group (Table 1).
Table 1. Anthropometric data and vital signs of the diabetes and control groups.
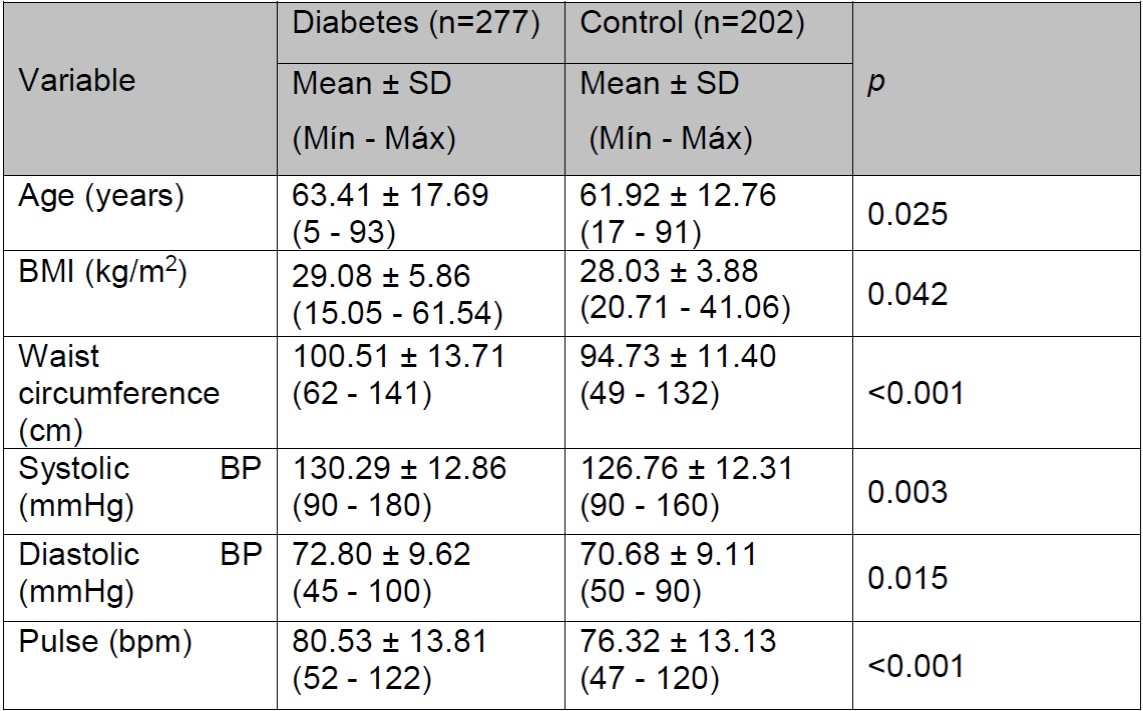
(SD, standard deviation; Min, minimum; Max, maximum; BMI, body mass index; BP, blood pressure.)
Breaking down the anthropometric data of the diabetes group into its subgroups, we found the following significant differences (p<0.050): greater age of the neuropathy, vasculopathy, and neurovasculopathy subgroups than the no pathology subgroup (p<0.001); although the body temperature is similar in all subgroups, that of the neurovascular subgroup is significantly greater than that of the no pathology subgroup (p=0.048); the BMI is greater in the neuropathy, vasculopathy, and neurovasculopathy subgroups than in the no pathology subgroup and the non-diabetics control group (p<0.001); and the waist circumference is greater in all the diabetes subgroups than in the non-diabetics control group (p<0.001) (Table 2).
Table 2. Anthropometric data of the control group and the disaggregated diabetes group.
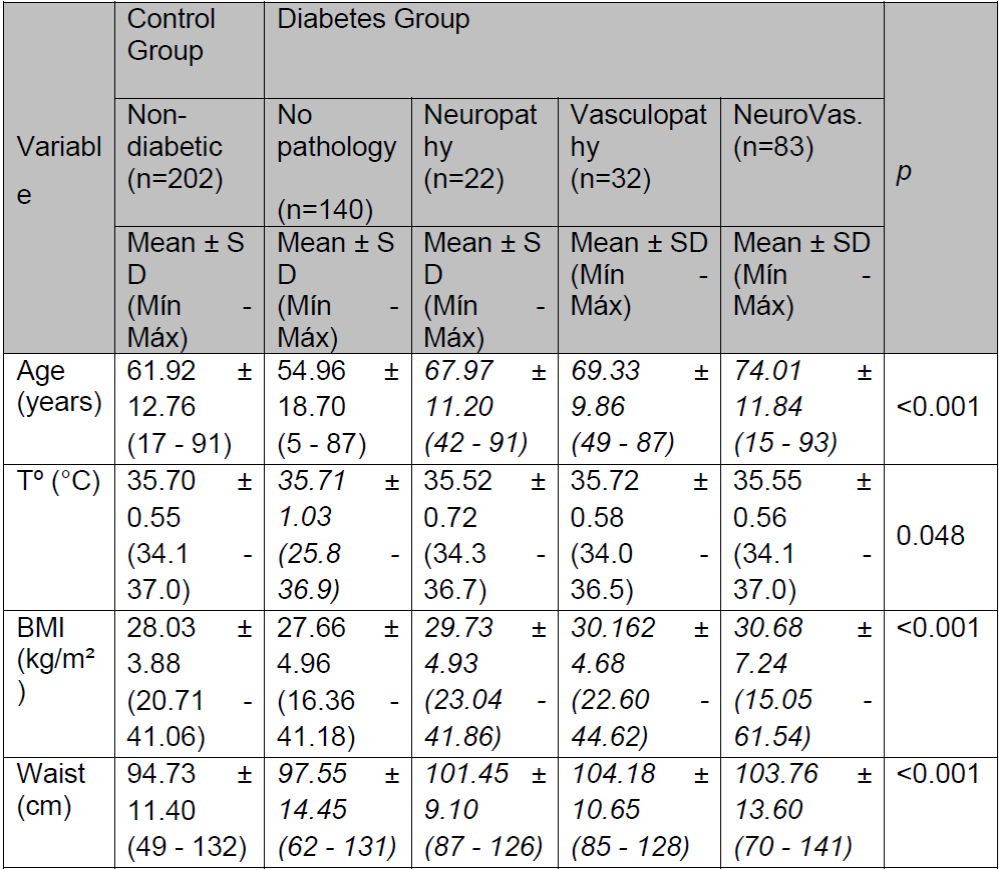
(SD, standard deviation; Min, minimum; Max, maximum; BMI, body mass index; NeuroVas., neurovasculopathy; Tº, temperature; Waist, waist circumference.)
For the descriptive statistics of the diagnosis and evolution of DM, on the date of collection of the information, there was a mean 16.89 years of evolution of the pathology, with a mean basal glycæmia of 138.96 mg/dL, 12.96 mg/dL above the value established as pathological (≥126-130 mg/dL). The glycosylated hæmoglobin was 7.32%, greater by a value of 0.82% than the criterion established for a diagnosis of DM (≥6.5%). The complete lipid profile met the criteria of normality: 166.61 mg/dL total cholesterol (<200 mg/dL), 52.38 mg/dL HDL cholesterol (>50 mg/dL), and 92.78 mg/dL LDL cholesterol (<100 mg/dL) (Table 3).
Table 3. Diagnosis and evolution of the diabetes group

(SD, standard deviation; HbA1c, glycosylated hæmoglobin.)
The values therefore follow an accepTable behaviour in accordance with the criteria of normality of the said parameters of the American Diabetes Association.
Comparing risk factors between the control (non-diabetic) group and the disaggregated categories of the diabetes group, we found the following significant differences (p<0.050): greater index of tobacco use in the no pathology subgroup compared to the non-diabetics control group (p=0.048); greater presence of cholesterol and HBP risk factors in the vasculopathy and neurovasculopathy subgroups than in the no pathology subgroup and non-diabetics control group (p<0.001); greater prevalence of ischæmic heart disease in the neurovasculopathy subgroup than the non-diabetic control group (p<0.001); and greater prevalence of obesity in all the diabetes subgroups than in the non-diabetics control group (p<0.001) (Table 4).
DISCUSSION
From the statistical study of the variables, one can affirm that the relationship between the different risk factors associated with the appearance of the diabetic foot was found to be significant. The first results on risk factors in the anthropometric data are clear in three specific variables - weight, BMI, and waist circumference.
The DM patients had notably greater values of weight (p=0.048), BMI (p=0.042), and waist circumference (p<0.001) than those of the control group. There also stands out the differences in the systolic (p=0.003) and diastolic (p=0.015) blood pressure data which were also lower in the control group.
The relevance of these data is supported by various studies that have associated cardiovascular risk factors such as overweight or obesity and arterial hypertension in diabetic patients with the development of diabetic neuropathy and the diabetic foot15.
In a study of 1172 DM-1 patients with no complications, Tesfaye recorded 276 to have developed some neuropathy during a seven-year follow-up evaluation. The cumulative incidence of neuropathy was related to the glycosylated hæmoglobin value and the duration of the diabetes, but, after adjusting for these factors, the authors found that greater body mass index, hypertension, and smoking were significantly related to the accumulated incidence of neuropathy15.
In the Tesfaye study, higher levels of total lipoproteins, cholesterol, and low density triglycerides were also considered to be risk factors15. But in the present study, the complete lipid profile met the criteria for normality with 166.61 mg/dL total cholesterol (<200 mg/dL), 52.38 mg/dL HDL cholesterol (>50 mg/dL), 92.78 mg/dL LDL cholesterol (<100 mg/dL), and 7.32% glycosylated hæmoglobin (≥6.5%).
Nevertheless, these data have to be interpreted from a sample perspective. In particular, while the present study included patients with both types of diabetes (DM-1 and DM-2), that of Tesfaye only included patients of Type 1. There could have been differences regarding the number of DM-2 patients who might have developed the pathology, with their risk factors influencing that development to a greater extent. Other differences in the Tesfaye study are the 1172 sample size, the 7-year follow-up, the evaluation method, and the non-differentiation by sex.
This last factor is especially pertinent because the assumption of the influence of sex on the development of foot ulcers has been somewhat controversial. Some studies have established the male sex as itself being a risk factor. On the one hand, men are more prone to having some of the key independent predictors, such as a greater prevalence of peripheral neuropathy16,17, and smoking and cardiovascular risk factors18,19. And on the other, research has shown that men are almost twice as likely as women to have sensory neuropathy, the commonest type of neuropathy associated with diabetic foot ulceration, and they have nerve conduction abnormalities that are more serious16,20.
This is in contrast with recent studies which have established a greater prevalence of diabetic peripheral neuropathy in women21,22. In our study, the sample of diabetic patients was 49.8% men and 50.2% women, but sex was not taken into account as an intervening variable.
Advanced age and longer than 10 years of evolution of DM are also important risk factors with respect to the development of complications associated with DM, such as the diabetic foot. In the present study, the mean age of patients with DM depended on the pathology or lack of pathology with which they were associated.
The mean age of the group with DM but no other associated pathology was 54.96 ± 18.70, and they represented more than 50% of the sample with DM. They were the youngest subgroup, younger even than the control group. The mean age of the subgroup of DM patients with neuropathy (8% of the sample) was 67.97 ± 11.20. The mean age of the subgroup with vasculopathy (11% of the sample) was 69.33 ± 9.86, and the subgroup with neurovascular complications (representing almost 30% of the sample) was the oldest with a mean age of 74.01 ± 11.84 years.
The data on age and the possible complications associated with DM are coherent with the findings of other studies. Rahman23 examined how the prevalence in DM-2 patients of various microvascular complications was related to anthropomorphic factors, identifying the clinical and biochemical characteristics linked to those complications. The results showed that the risk factor for developing any form of microvascular complication was advanced age, with the age of those with diabetic neuropathy being greater (63.3 ± 13.1 years), a Figure just 4 years away from that observed in the present study. Age as a risk factor for diabetic complications had already been confirmed by previous work24. The mean age of our control group was 61.92 ± 12.76.
With respect to BMI, it is important to note that, although as mentioned above, the BMI of the group with DM was greater overall than that of the controls, there were noTable differences among the DM subgroups.
The lowest BMI (even including the controls) was that of DM patients with no associated pathology - 27.66 ± 4.96. This was followed closely (by less than half a unit) by the control group with 28.03 ± 3.88. This may just have been a reflection of the age difference between the two since the no pathology subgroup had the lowest mean age of all. The surprising thing is that the situation is inverted for the waist circumference data, this being 94.73 ± 11.40 for the control group and 97.55 ± 14.45 for the DM with no pathology subgroup.
All the rest of the groups had a greater BMI correlated with greater waist circumference compared to the control group. But to what extent this may be a predictor of complications or of the diabetic foot is unclear. Different studies have specifically linked high values of BMI and waist circumference to DM-225,26,27, but some researchers give more value to one measurement over the other. Flegal for example, argues for the use of waist circumference as a measure of obesity to predict risk to health. Among the arguments given are: that waist circumference has shown itself to be a good or even better predictor than BMI of metabolic syndrome, diabetes, cardiovascular disease, and all-cause mortality; that it provides information about health risk in addition to that provided by the BMI; and that it is conceptually easy to measure, although it requires some training and standardization28.
Others, however, have noted that replacing BMI by waist circumference as an indicator of the risk of cardiovascular disease and diabetes may be an over simplification29. Some of the counterarguments are: that waist circumference is strongly correlated with BMI30.
That it does not differentiate between subcutaneous fat and visceral fat; that it remains to be demonstrated that there is a consistent association between waist circumference and visceral fat after adjusting for age and BMI; and that the distribution of body fat is different in the strata of race, sex, and age.
CONCLUSIONS
One can conclude by stating that the study of the different risk factors is key to the diagnosis of the foot at risk. It can be clearly established that age is a determining factor, since advanced ages correspond to greater BMI and waist circumference. Together with the analysis by infrared thermography in the evaluation of the foot at risk, a risk factor study is useful for the diagnosis and prevention of compromised areas of the foot, thus avoiding the evident triggering of the damage typical of a diabetic foot. In these patients, prevention is essential to improve their quality of life, while at the same time reducing the health service costs inherent in the expensive treatments of the complications of a diabetic foot.
REFERENCIAS
1. Organización Mundial de la Salud. Global Status Report on noncommunicable diseases 2014 [Internet]. Suiza; 2014 [citado 20 de febrero de 2017]. 302 p. Recuperado a partir de: http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf [ Links ]
2. Home P, Mant J, Diaz J, Turner C, Guideline Development Group. Management of type 2 diabetes: summary of updated NICE guidance. BMJ BMJ Group; 2008;336(7656):1306-8. [ Links ]
3. SED Sociedad Española de Diabetes. Tratado de Diabetes Mellitus. 2a. Editoria medica panamericana, editor. Madrid; 2017. 732 p. [ Links ]
4. Faris I. Mechanisms for the development of foot lesions. En: Churchill Livingstone, editor. The management of the Diabetic Foot. 2a ed. London: Elsevier Health Sciences; 1991. p. 5-8. [ Links ]
5. Hildebrandt C, Zeilberger K, John Ring EF, Raschner C. The Application of Medical Infrared Thermography in Sports Medicine. En: An International Perspective on Topics in Sports Medicine and Sports Injury. InTech; 2012. [ Links ]
6. Astasio-Picado A, Escamilla Martínez E, Martínez Nova A, Sánchez Rodríguez R, Gómez-Martín B. Thermal map of the diabetic foot using infrared thermography. Infrared Phys Technol Pergamon; 2018;93:59-62. [ Links ]
7. Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. 1948. J Appl Physiol. 1998;85(1):5-34. [ Links ]
8. Bagavathiappan S, Saravanan T, Philip J, Jayakumar T, Raj B, Karunanithi R, et al. Infrared thermal imaging for detection of peripheral vascular disorders. J Med Phys Medknow Publications; 2009;34(1):43-7. [ Links ]
9. Armstrong DG, Lavery LA, Liswood PJ, Todd WF, Tredwell JA. Infrared dermal thermometry for the high-risk diabetic foot. Phys Ther. 1997;77(2):169-75; discussion 176-7. [ Links ]
10. American Diabetes Association. Standards of Medical Care in Diabetes-2017: Summary of Revisions. Diabetes Care. 2017;40(Supplement 1):S4-5. [ Links ]
11. Argimon JM, Jiménez J. Métodos de investigación clínica y epidemiológica. 4a ed. Barcelona: Elsevier; 2013. [ Links ]
12. Ammer K. The Glamorgan Protocol for recording and evaluation of thermal images of the human body. Appendix II: Regions of interest. Thermol Int. 2008;4:136-44. [ Links ]
13. Chao CC, Hsieh SC, Yang WS, Lin YH, Lin WM, Tai TY, et al. Glycemic control is related to the severity of impaired thermal sensations in type 2 diabetes. Diabetes Metab Res Rev. 2007;23(8):612-20. [ Links ]
14. Malterud K. Qualitative research: standards, challenges, and guidelines. Lancet. 2001;358(9280):483-8. [ Links ]
15. Tesfaye S, Chaturvedi N, Eaton SEM, Ward JD, Manes C, Ionescu-Tirgoviste C, et al. Vascular Risk Factors and Diabetic Neuropathy. N Engl J Med. 2005;352(4):341-50. [ Links ]
16. Kiziltan ME, Gunduz A, Kiziltan G, Akalin MA, Uzun N. Peripheral neuropathy in patients with diabetic foot ulcers: Clinical and nerve conduction study. J Neurol Sci. 2007;258(1-2):75-9. [ Links ]
17. de Souza RJ, de Souza A, Nagvekar MD. Nerve conduction studies in diabetics presymptomatic and symptomatic for diabetic polyneuropathy. J Diabetes Complications. 2015;29(6):811-7. [ Links ]
18. Townsend RR, Machin I, Ren J, Trujillo A, Kawaguchi M, Vijapurkar U, et al. Reductions in Mean 24-Hour Ambulatory Blood Pressure After 6-Week Treatment With Canagliflozin in Patients With Type 2 Diabetes Mellitus and Hypertension. J Clin Hypertens. 2016;18(1):43-52. [ Links ]
19. Tan YY, Gast G-CM, van der Schouw YT. Gender differences in risk factors for coronary heart disease. Maturitas. 2010;65(2):149-60. [ Links ]
20. Lee HC, An SG, Lee HW, Park J-S, Cha KS, Hong TJ, et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J. 2012;76(7):1750-60. [ Links ]
21. Kandil MR, Darwish ES, Khedr EM, Sabry MM, Abdulah MA. A community-based epidemiological study of peripheral neuropathies in Assiut, Egypt. Neurol Res. 2012;34(10):960-6. [ Links ]
22. Kim SS, Won JC, Kwon HS, Kim CH, Lee JH, Park TS, et al. Prevalence and clinical implications of painful diabetic peripheral neuropathy in type 2 diabetes: Results from a nationwide hospital-based study of diabetic neuropathy in Korea. Diabetes Res Clin Pract Elsevier; 2014;103(3):522-9. [ Links ]
23. Rahman M, Ford CE, Cutler JA, Davis BR, Piller LB, Whelton PK, et al. Long-Term Renal and Cardiovascular Outcomes in Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Participants by Baseline Estimated GFR. Clin J Am Soc Nephrol. 2012;7(6):989-1002. [ Links ]
24. Mørkrid K, Ali L, Hussain A. Risk factors and prevalence of diabetic peripheral neuropathy: A study of type 2 diabetic outpatients in Bangladesh. Int J Diabetes Dev Ctries. 2010;30(1):11. [ Links ]
25. Pérez A, Franch J, Cases A, González Juanatey JR, Conthe P, Gimeno E, et al. Relación del grado de control glucémico con las características de la diabetes y el tratamiento de la hiperglucemia en la diabetes tipo 2. Estudio DIABES. Med Clin (Barc). Elsevier Doyma; 2012;138(12):505-11. [ Links ]
26. Hsu WC, Araneta MRG, Kanaya AM, Chiang JL, Fujimoto W. BMI Cut Points to Identify At-Risk Asian Americans for Type 2 Diabetes Screening: Table 1. Diabetes Care. 2015;38(1):150-8. [ Links ]
27. Cheon DY, Kang JG, Lee SJ, Ihm SH, Lee EJ, Choi MG, et al. Serum Chemerin Levels are Associated with Visceral Adiposity, Independent of Waist Circumference, in Newly Diagnosed Type 2 Diabetic Subjects. Yonsei Med J. Yonsei University College of Medicine; 2017;58(2):319-25. [ Links ]
28. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of All-Cause Mortality With Overweight and Obesity Using Standard Body Mass Index Categories. JAMA. 2013;309(1):71. [ Links ]
29. Romaguera D, Ängquist L, Du H, Jakobsen MU, Forouhi NG, Halkjær J, et al. Dietary Determinants of Changes in Waist Circumference Adjusted for Body Mass Index a Proxy Measure of Visceral Adiposity. Calbet JAL, editor. PLoS One [Internet]. 2010;5(7):e11588. [ Links ]
30. Kapil U, Sachdev HP. Urgent Need to Orient Public Health Response to Rapid Nutrition Transition. Indian J Community Med. 2012;37(4):207. [ Links ]
Received: October 24, 2018; Accepted: January 21, 2019











 texto en
texto en 



