My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Enfermería Global
On-line version ISSN 1695-6141
Enferm. glob. vol.20 n.62 Murcia Apr. 2021 Epub May 18, 2021
https://dx.doi.org/10.6018/eglobal.443241
Originals
Laser-acupuncture to control blood glucose in Type II Diabetes: a randomized clinical trial
1 PhD. Technician in Public Health from the Fernandes Figueira National Institute for Child, Female and Adolescent Health. Osvaldo Cruz Foundation. Rio de Janeiro, RJ, Brazil
2PhD. Head Professor at the Department of Fundamental Nursing of the Anna Nery Nursing School/Federal University of Rio de Janeiro (DEF/EEAN/UFRJ). Rio de Janeiro, RJ, Brazil.
3PhD. Adjunct Professor I at the Nursing Graduate Course of the University of Vassouras. Vassouras, RJ, Brazil.
4PhD. Professor at the Nursing and Obstetrics Course of the UFRJ Campus/Macaé. Macaé, RJ, Brazil
5PhD. Biomedical Engineering Program PEB/Coppe of the Federal University of Rio de Janeiro. Clinical Research Unit of the Fernandes Figueira Institute/Fiocruz. Rio de Janeiro, RJ, Brazil
6Doctoral student. Subistitute Professor at the Public Health Department of the Anna Nery Nursing School/Federal University of Rio de Janeiro (EEAN/UFRJ). Rio de Janeiro, RJ, Brazil
Objective
To evaluate the effectiveness of laser-acupuncture applied to nursing care for people with type II diabetes mellitus.
Methods:
Randomized, triple-blinded clinical trial, with 42 type II diabetic participants, both genders, aged between 30 and 75 years, using oral hypoglycemic agents and difficulties in maintaining postprandial glycaemia ≤ 180mg/dl. Participants were randomly assigned to two different groups and submitted to standard laser-acupuncture, experimental arm, or simulated, control arm. Descriptive and association analyses between variables were used, with a significance level of 5% (p <0,05). To compare blood glucose results, Student’s t test was used for paired samples and Anova for repeated measurements.
Results:
A significant decrease in capillary glycaemia was observed in the participants in the experimental arm, a phenomenon not verified in those in the control arm.
Conclusions:
the plausibility of using the technique as a technology for nursing care for people with type II diabetes mellitus is inferred. Brazilian Registry of Clinical Trials. UTN: U1111-1181-1675. Clinical Trials NCT02605889.
Key words: Nursing Care; Diabetes Mellitus, Type 2; Complementary Therapies; Acupuncture Therapy
INTRODUCTION
Type II diabetes mellitus (DM-II) is a chronic noncommunicable disease (CNCD) characterized by persistent hyperglycemia due to insulin resistance or by decreased secretion1. It is not a single disease, but a heterogeneous group of metabolic disorders that presents hyperglycemia in common, resulting from defects in the action and/or secretion of insulin2.
In 2005, 240 million people in the world had DM, representing an increase of 105 million over 20 years3. By 2030, the disease shall reach 366 million people, and two-thirds will live in developing countries3,4.
In the search for the control and prevention of CNCD, Brazil has been working with technical and strategic alliances with different bodies, such as the Ministry of Health (MH) and its health care institutions, Scientific Societies, Universities and Non-Governmental Organizations (NGOs) in partnership with the Southern Common Market (MERCOSUR), in addition to interinstitutional partnerships with the Pan-American Health Organization (PAHO), the World Health Organization (WHO) and the Joint Network of Actions for the Reduction and Management of Noncommunicable Diseases (CARMEN Network)5.
The persistence of high circulating glucose levels in the bloodstream causes micro and macro vascular lesions that over the years of exposure can lead to retinopathies, chronic kidney disease and skin lesions, amputation of the lower limbs or part of them. These complications compromise quality of life and generate early work disabilities, leading to increased costs to the Unified Health System (UHS) and the social security system6,7.
The treatment of the disease aims to avoid changes in blood glucose rates and considers a combination of pharmacological methods, changes in eating and behavioral habits1,2.
Acupuncture (AP) and other techniques of Traditional Chinese Medicine (TCM) have also been used as a therapeutic proposal for DM.
Used for thousands of years for the treatment of this disease and other associated conditions, AP has been recommended by the WHO since 2002, as complementary treatment to the health of people with DM, with an indication of a reduction of up to 20% of blood glucose levels by endocrine stimulation, besides contributing to the control of stress, obesity, hyperphagia, hyperlipidemia, inflammation, altered activity of the sympathetic nervous system and insulin signaling defects, favoring immunity, and the protection of organs targeted by the effects of hyperglycemia8.
The AP is a technique of the TCM, practiced for millennia aiming to promote the harmonization of energies for health production/preservation, through the stimulation of specific points of the body called acupoints9,10.
Among its application techniques is Laser acupuncture (LA), which does not use needles in its approach, as occurs in the traditional method of AP, using low intensity laser directly on the skin in the acupoints9,10.
LA has significant efficacy when compared to other AP methods and has the main advantages of being a rapid treatment, considering the length of stay of the patient in therapy, and a very low risk of local infection, considering that it is not an invasive procedure11.
The AP is able to integrate the context of nursing care into the quality of innovative technology, expanding the possibilities of the professional’s therapeutic action9. However, there is a lack of studies with experimental designs demonstrating the scientific evidence of the efficacy of AP to DM-II and pointing out its limits and possibilities as care technology, providing care safety12.
Thus, the objective of this research was to evaluate the efficacy of laser-acupuncture applied to nursing care for people with type II diabetes mellitus.
MATERIAL AND METHOD
Randomized, multicenter, triple-blind, two-arm clinical trial with random allocation rate of fifty percent per arm. The study was developed in three research centers in two states of the Southeastern Brazilian region: in the service laboratory of the Integrated Research-Assistance Program (PIPA), Anna Nery Nursing School/Federal University of Rio de Janeiro (EEAN/UFRJ), Rio de Janeiro/RJ, research coordinating center; and in basic health units, linked to the health departments of the city of Vitória/ES, and Maricá/RJ, as participating centers.
The research received funding from the Ministry of Science, Technology and Innovation/National Council for Scientific and Technological Development/Ministry of Health (MCTI/CNPq/MS), through edict n. 007/2013, which enabled logistics, the acquisition of materials and the displacement of the research team.
The sample size was calculated in observance of the results of a meta-analysis study on the theme(13) due to the proximity to the proposed study design. Using these data and considering a confidence level of 95% and a power of 80%, the sample size to identify significant differences of the same magnitude in the study resulted in 148 participants, distributed equally between the intervention and control arms. Nevertheless, given the difficulty to recruit the participants, a calculation of the power of the sample was performed after data collection with 42 participants (21 in each arm). This was considered satisfactory, maintaining the confidence level of 95%, power of 80% and expected average reduction of PPG before and after exposure of 29.9 (SD±68.3).
Therefore, the sample consisted of 42 participants who met the following inclusion criteria: people with DM-II, of both sexes, aged 30 to 75 years, using oral hypoglycemic medication for one year or more and with difficulties in maintaining postprandial glucose at levels ≤ 180mg/dl. Those who presented conditions that could interfere with glucose metabolism and its processes involved were excluded from the study, including: insulin-dependent, smokers, alcohol consumers, in drug treatment for other diseases, including obesity, using a specific diet for weight loss, practitioners of regular physical activity, patients with neoplasms and pregnant women, as well as individuals with amputation in any of the lower limbs or part of them, depending on the location of the acupuncture points selected for the intervention.
A pre-selection was performed by nurses and physicians working in the care units of the research centers, through the inclusion and exclusion criteria previously exposed to the team. The pre-selected patients were invited to participate in the research through telephone contact, scheduling the initial nursing consultation, performed by an evaluator nurse, assisting in the research.
This nursing consultation was performed by a nurse, a member of the research team, with the objective of clarifying the purpose of this study, including ethical issues; analyzing the patient’s profile for inclusion in the study; and defining the initial nursing diagnoses. Those who met the chosen inclusion criteria read and signed the Informed Consent Form. Those who refused to participate in the study or who did not meet the inclusion criteria received the appropriate guidance and referrals regarding the health problems detected.
A research assistant was responsible for the registration of participants through an alphanumeric identification code and the use of the random allocation technique by blocks in the test groups, being Arm A - intervention (experimental) and B - Control (simulated), using outsourced randomization service available from the Internet (http:// www.sealedenvelope.com).
In order to comply with the proposed methodological rigor, a single treatment protocol was used that could benefit all participants. This protocol was formulated by the researcher through the study of the available literature on the disease, based on the complaints and main nursing diagnoses identified in patients with DM-II, making a correlation with the energetic diagnosis of TCM.
The participants selected for arm A underwent the laser-acupuncture procedure. This consists of using the stimulation of acupuncture points through a low-power laser, using an infrared laser-acupuncture equipment of gallium and aluminum arsenide (Ga-Al-As), with the following technical specifications: infrared 6MW of power, with frequency of Nogier and IR receptor test. Those selected for arm B underwent a simulated procedure, using a device identical to the LA (control), but without the laser light emission diode. This device was produced by the manufacturer of the LA device, at the request of the researcher for the simulation to be performed. The device retained the same external characteristics, preventing the participant, researcher and evaluator nurse from identifying it. A research assistant throughout the study was responsible for keeping and preparing the equipment according to the allocation arm of each participant. Only she knew the right equipment for intervention and simulation. This process was essential for the triple blinding condition promoted by the study. The participant’s location in the research arm was only revealed to those involved after the completion of all stages of data collection.
Each point was approached bilaterally, with the laser emission device positioned under the participant’s skin, directed 45º in the direction of fluency of the meridian in question, for 90 seconds at each point, in this sequence: BP3 (Taibai), BP6 (Sanyinjiao), E36 (Zusanli), at the frequency of 10Hz; and B20 (Pishu) and M-DC12 (YiShu) at the frequency of 20Hz.
The treatment protocol also occurred for both arms of the study, with six 25-minute meetings between preparation and procedure, with a weekly interval, totaling six weeks of treatment.
The expected outcome was the reduction of postprandial glucose to levels below 180md/mL. Thus, the research assistant performed the capillary glucose collection before and after the sessions, following a standardized collection protocol for the study. The glucometer used has the principle of measurement by photometric determination of glucose using glucose-colorant-oxidoreductase (synonym: reaction of the mediator of PQQ-dependent glucose dehydrogenase), with the variation of the measurement from 10 mg/dl to 600 mg/dl or 0.6 mmol/L - 33.3 mmol/L. The reactive tapes were compatible with the device with Mut Q-GDH specification = glucose dehydrogenase with modified pyrolquinolnoquinone to avoid interference with maltose (ANVISA Record: 10287411023). For capillary puncture, disposable lancets were used, following ISO 13485 and NR32 of the Ministry of Labor and Employment.
In order to homogenize the sample, the participants were instructed to keep fasting 2 hours before the procedure. The results were under the responsibility of the research assistant who proceeded to randomize and prepare the material, being recorded in the participants’ form, transcribed to a Microsoft Excel spreadsheet and later transferred to the statistical analysis software. The data were kept confidential for both the participants and the researcher until the end of data collection.
The data were processed by descriptive analyses and association between variables, obeying a significance level of 5% (p<0.05), using Statistica software version 12.0. For the comparison between the blood glucose results, the Student’s t-test was used for paired samples and Anova for repeated measurements.
The study was approved by the Research Ethics Committee (REC) of the Ana Nery Nursing School/São Francisco de Assis Hospital, of the Federal University of Rio de Janeiro, opinion n. 772.509, registered for the international public domain in the Clinical Trials (clinicaltrials.gov), available through the identifier NCT02605889, and on the website of the Brazilian Registry of Clinical Trials (ReBEC) under the UTN Number: U1111-1181-1675.
RESULTS
Participants were recruited in September and November 2014 and June 2015. Data were collected from October 3, 2014 to January 9, 2015, at the research-coordinating center (EEAN/UFRJ) and in the health units of the participating center of Vitória/ES; and from July 30, 2015 to September 3, 2015, at the participating center Maricá/RJ.
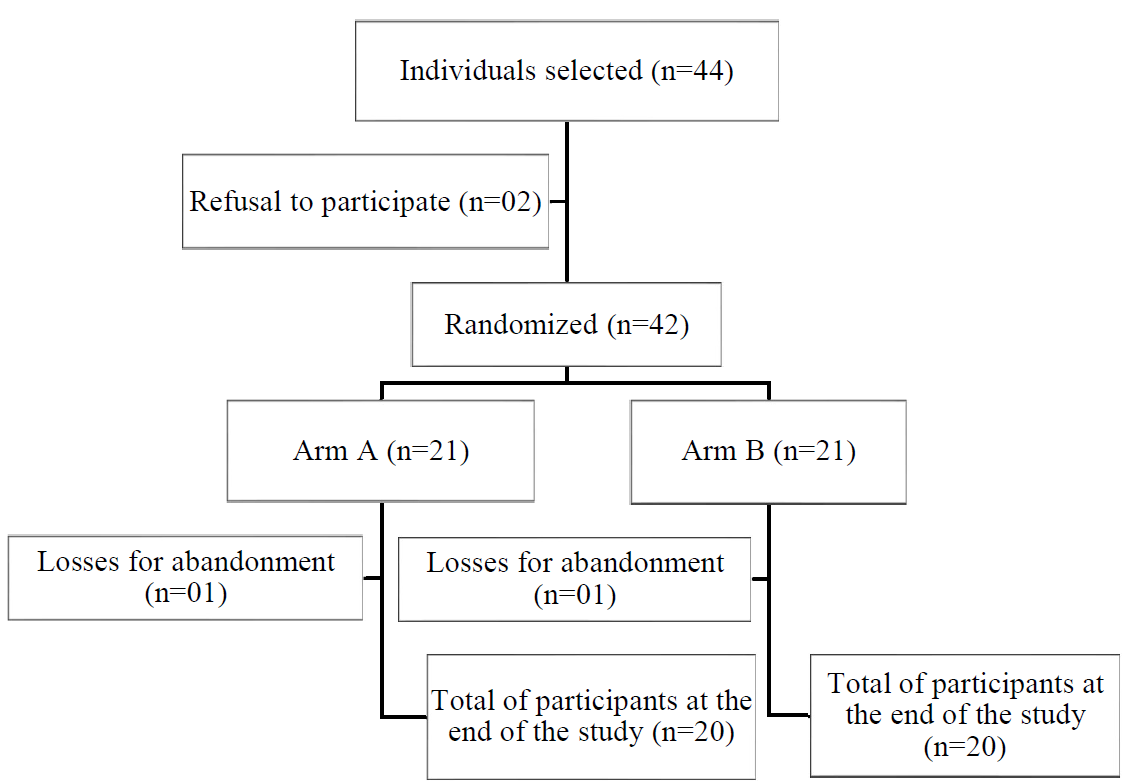
Forty-four individuals were selected, but 42 agreed to participate in the research, signing the ICF, composing the final sample size. This was considered satisfactory with a confidence level of 95%, power of 80% and expected average reduction of PPG before and after exposure of 29.9md/mL (SD±68.3), a fact that indicated that the collection could be closed without impairing the results. The difficulties were due to various reasons, from failures in the registration of patients in health units to the locomotion of individuals to health units.
There was a loss of two participants (4.7% of the sample) who did not attend the final consultation (6th meeting), one from each arm. However, as there was no withdrawal of the ICF authorization, their data were kept in the study. Each testing arm was also formed by 50% of the sample, 21 participants. Arm A received the experimental procedure and arm B, the placebo procedure (Figure 1).
The median exposure was between 5 and 6 exposures, with the female gender as the most sTable, whose mean was 4.7 exposures and standard deviation of 1.1 and 1.2, respectively. The total average exposure was 4.9 and standard deviation was 1.0 (Table 1).
Table 1. Distribution profile of the participants in the research. Rio de Janeiro, RJ, Brazil, 2015.

* Number of participants † Standard deviation
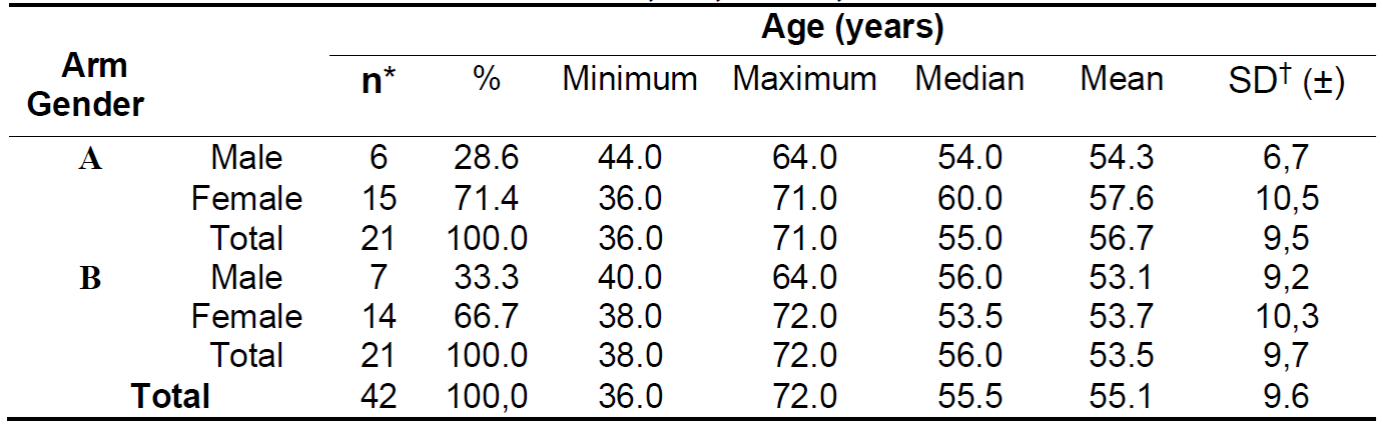
The general sample was characterized by a majority of female participants (69.0%, n = 29), aged 55.7 and SD ± 10.4 years. The mean overall age was 55.1 years and SD ± 9.6 (Table 2).
Table 2. Distribution profile of participants by arm, gender and age. Rio de Janeiro, RJ, Brazil, 2015.
* Number of participants † Standard deviation
Arm A 71.4% (n = 15) consisted of women with a mean age of 57.6 years (SD ± 10.5) and median of 60.0 years. The men in this arm totaled 28.6% of the sample (n = 6) with mean and median age in 54.3 (SD ± 6.7) and 54 years, respectively.
In arm B, women represented 66.7% of the sample (n = 14) and men, 33.3% (n = 7), with a mean age for men of 53.1 (SD ±9.2) and 53.7 (SD ± 10.3) for women (Table 2).
Metformin was the most used medication between arms A and B, totaling 54.76% of the participants. The use of Glibeclamide was presented by 11.90%. The combined use of these two drugs totaled 33.33%.
The overall mean pre-exposure PPG value of the participants was 216.39 mg/dL (SD ± 79.00 mg/dL), being 229.32 mg/dL in arm A and 201.94 in arm B (Table 3).
Table 3. Initial blood glucose value. Rio de Janeiro, RJ, Brazil, 2015.

*Obtained by postprandial capillary puncture † Standard deviation
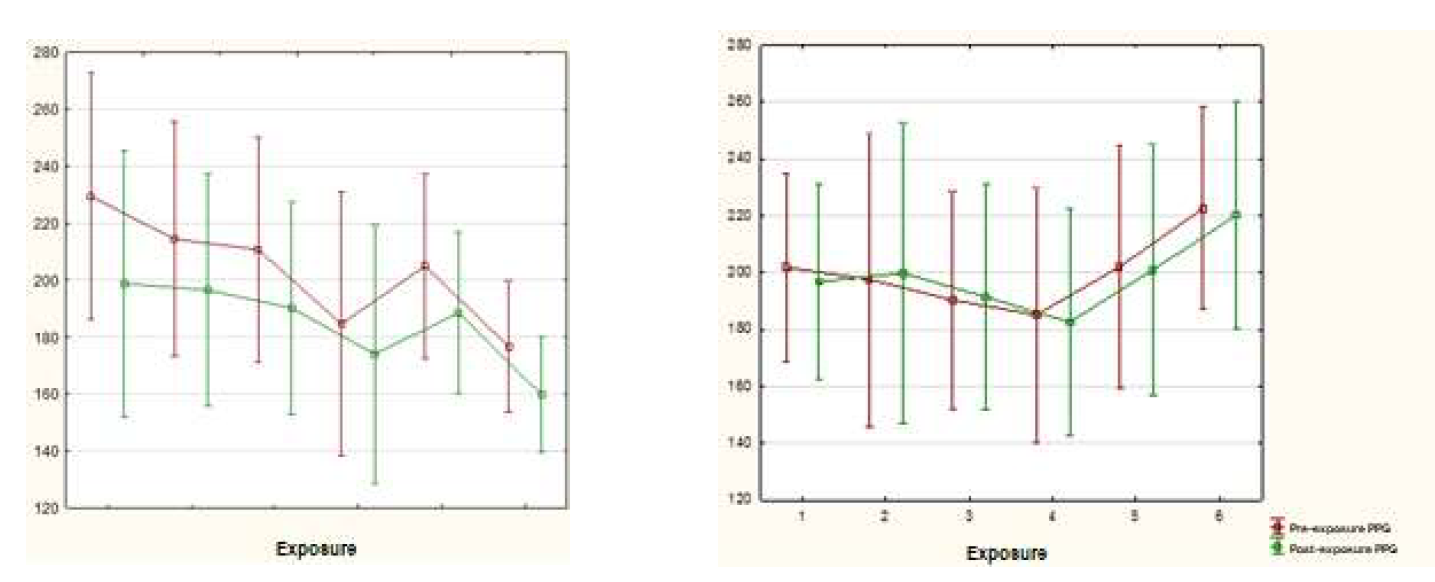
During the exposures, the behavior of the variability of the PPG between the two testing arms was observed. In arm A, the means showed a reduction, differently from arm B (Graph 1).
In arm A, there were statistically significant differences before and after exposure for
all moments considered. Unlike arm B, which did not present significant differences for any of the moments (Table 4).
Table 4. Analysis of the behavior of the PPG throughout the exposures. Rio de Janeiro, RJ, Brazil, 2015

*Postprandial glucose **p-value obtained by Student’s t test for paired samples † Standard deviation
The efficacy of the intervention in arm A was proven after analysis of the mean PPG in before exposure compared to after exposure, in which it presented a reduction in values from 229.32 to 174.95 (p=0.003). On the other hand, the participants of arm B presented a slightly higher average in the 6th post-exposure, but not enough for the test to differentiate it from that of the 1st pre-exposure (Table 5).
DISCUSSION
The results obtained revealed the existence of efficacy of LA, with the proposed protocol, in nursing care for people with DM-II, as complementary to drug treatment, assisting in the acute reduction of postprandial hyperglycemia, considering its better control and less variation.
However, it is not possible to affirm the long-term maintenance of this effect, or even after interrupting the therapy, considering that there was no follow-up of the glycemic profile of the participants after its interruption, which is an important limitation of the research.
It is important to point out that the period of data collection coincided with the Christmas and end-of-year festivities, when people usually do not regulate the consumption of food and beverages. It is believed that this fact may be related to the lower fall in glycemic levels of the participants presented at the end of the collection period sampled by arm A, as well as the increased behavior in this same period shown by arm B.
The findings in this study corroborate those of other studies that investigated the effects of AP in the treatment of people with diabetes, not only in the reduction of PPG, but also considering the correction of several metabolic disorders such as overweight, hyperphagia, hyperlipidemia, inflammation, insulin secretion disorders and in the treatment of peripheral neuropathy, bladder dysfunction or symptoms of other comorbidities that did not respond to conventional therapy13)(14)(15)(16.
A pilot study that evaluated the effect of single-point acupuncture (VC 12 - Zhongwan) on blood glucose levels in healthy volunteers showed a slight reduction in random capillary glucose levels in the acupuncture group and a slight increase in the placebo control group, as well as in the present study17.
It is known that there is a close correlation of persistent hyperglycemia in diabetics with macro and micro vascular injuries that will lead to irreversible lesions in the glomerular filtration system, optic and peripheral nerves18)(19)(20)(21)(22.
In this context, it is possible to affirm that LA can contribute to the acute decrease of these conditions in view of the observed reduction in the glycemic levels of the participants. Thus, LA is an effective non-pharmacological option in the care of diabetic people, in both blood glucose control and other factors related to the integrality of care to the human being.
Among the challenges presented in the development of this study, perhaps the main one was to overcome the limits imposed by biomedical science in the construction of evidence about the efficacy of LA in human health. This is because its interventional patterns, of Cartesian nature, differ from those that provide identity to integrative and complementary practices, the vitalist ones. Thus, in principle, the need to create a single protocol for all participants is in line with the singularity proposed by LA, since it considers the responses of the organism to internal and external factors understood as aggressors.
That said, the intervention protocol used in this study was carefully thought out, in view of the fact that clinical research requires the performance of a single procedure between the arms so that the answers can be correlated with each other.
CONCLUSION
The results of this research found the reduction and acute control of capillary glycaemia through the proposed LA protocol, demonstrating the potential of this therapy applied to nursing care for with type II diabetes mellitus.
For greater deepening and understanding of the results and their clinical benefits after interrupting the therapy, new studies should be developed. It is necessary to work on the possible and necessary dialogue between the biomedical model, dominant in health, and the vitalist, emerging in this field, in favor of the support and legitimation of the use of AP in the professional field. It is urgent to think about the integration of different concepts and methods of care as a way to bring benefits to the person cared for.
REFERENCIAS
1. Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Básica. Estratégias para o cuidado da pessoa com doença crônica: diabetes mellitus. Brasília: Ministério da Saúde, 2013. Disponível em: http://bvsms.saude.gov.br/bvs/publicacoes/estrategias_cuidado_pessoa_diabetes_mellitus_cab36.pdf [ Links ]
2. Milech A, Oliveira JEP, Vencio S. Diretrizes da Sociedade Brasileira de Diabetes (2015-2016). São Paulo: A.C. Farmacêutica, 2016. Disponível em: https://www.diabetes.org.br/profissionais/images/docs/DIRETRIZES-SBD-2015-2016.pdf [ Links ]
3. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: 04estimates for the year 2000 and projections for 2030. Diabetes care. 2004; 27(5):1047-1053. doi: http://dx.doi.org/10.2337/diacare.27.5.1047 [ Links ]
4. Barceló A, Aedo C, Rajpathak S, Robles S. The cost of diabetes in Latin America and the Caribbean. Bull W.H.O. 2003; 81(1):19-27. Disponível em: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2572319/pdf/12640472.pdf [ Links ]
5. Brasil. Organização Pan-Americana da Saúde. Cooperação Técnica em DCNT no Brasil. Brasília: OPAS/OMS, 2020. Disponível em: https://www.paho.org/bra/index.php?option=com_content&view=article&id=573:cooperacao-tecnica-em-dcnt-no-brasil&Itemid=463 [ Links ]
6. Grossi SAA, Pascali PM. Manual de cuidados de enfermagem em Diabetes Mellitus. São Paulo: SBD; 2009. [ Links ]
7. Ferreira LT, Saviolli IH, Valenti VE, Abreu LC. Diabetes melito: hiperglicemia crônica e suas complicações. Arq bras ciênc saúde. 2011; 36(3):182-188. doi: http://dx.doi.org/10.7322/abcs.v36i3.599 [ Links ]
8. Peplow PV. Topical issue: acu-obesity and diabetes. J acupunct meridian stud. 2016; 9(3):107-108. doi: http://dx.doi.org/10.1016/j.jams.2016.01.009 [ Links ]
9. Pereira RDM, Alvim NAT. Theoretical and philosophical aspects of traditional chinese medicine: acupuncture, and diagnostic forms their relations with the care of nursing. Rev enferm UFPE on line. 2013; 7(1):279-288. doi: http://dx.doi.org/10.5205/reuol.3049-24704-1-LE.0701201336 [ Links ]
10. Pereira RDM, Alvim NAT. Acupuncture as a technology for intervention to nursing diagnosis. Rev enferm UFPE on line. 2016; 10(4):1286-1291. doi: http://dx.doi.org/10.5205/1981-8963-v10i4a11115p1286-1291-2016 [ Links ]
11. Alvim NAT, Pereira RDM, Pereira CD, Gomes Junior SCS, Bergold LB. Laser-acupuncture in nursing care for hypertensive individuals in primary care: case report. REME rev min enferm. 2017; 21:e-1035. doi: http://dx.doi.org/10.5935/1415-2762.20170045 [ Links ]
12. Pereira RDM, Alvim NAT, Pereira CD, Junior SCG. Acupuncture in hypertension and your contributions about nursing diagnoses. Esc. Anna Nery Rev Enferm. 2017; 21(1):e20170024. doi: http://dx.doi.org/10.5935/1414-8145.20170024 [ Links ]
13. Ingle PV, Samdani NR, Patil PH, Pardeshi MS, Surana SJ. Application of acupuncture therapy in type 2 Diabetes Mellitus patients. Pharma sci monit. 2011; 2(1):18-26. Disponível em: https://www.researchgate.net/publication/325009989_Application_of_Acupuncture_Therapy_in_Type_2_Diabetes_Mellitus_Patients#:~:text=A%20significant%20decreased%20were%20observed,with%20type%202%20diabetes%20mellitus. [ Links ]
14. Liang F, Koya D. Acupuncture: is it effective for treatment of insulin resistance? Diabetes obes metab. 2010; 12(7):555-569. doi: http://dx.doi.org/10.1111/j.1463-1326.2009.01192.x [ Links ]
15. Redmer J, Longmier E, Wedel P. Targeting diabetes: the benefits of an integrative approach. J fam pract. 2013; 62(7):337-344. Disponível em: https://cdn.mdedge.com/files/s3fs-public/Document/September-2017/6207_JFP_Article1.pdf [ Links ]
16. Gross JL, Ferreira SRG, Oliveira JE. Glicemia pós-prandial. Arq bras endocrinol metab. 2003; 47(6):728-738.doi:http://dx.doi.org/10.1590/S0004-27302003000600017. [ Links ]
17. Mohanty S, Mooventhan A, Manjunath NK. Effect of needling at CV-12 (Zhongwan) on blood glucose levels in healthy volunteers: a pilot randomized placebo controlled trial. J acupunct meridian stud. 2016; 9(6):307-310. doi: http://dx.doi.org/10.1016/j.jams.2016.08.002 [ Links ]
18. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 321(7258):405-412. doi: http://dx.doi.org/10.1136/bmj.321.7258.405. [ Links ]
19. Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005; 54(1):1-7. doi: tp://dx.doi.org/10.2337/diabetes.54.1.1. [ Links ]
20. Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141(6):421-431 doi: http://dx.doi.org/10.7326/0003-4819-141-6-200409210-00007. [ Links ]
21. Milech A, Chacra AR, Kayath MJ. Revisão da hiperglicemia pós-prandial e a hipoglicemia no controle do Diabetes Mellitus: o papel da insulina lispro e suas pré-misturas nos picos e vales. Arq bras endocrinol metab. 2001; 45(5):423-432. doi: http://dx.doi.org/10.1590/S0004-27302001000500004 [ Links ]
22. Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000; 23(Suppl2):B21-29. Disponível em: http://journal.diabetes.org/diabetescare/FullText/Supplements/DiabetesCare/Supplement400/B21.asp [ Links ]
Received: September 08, 2020; Accepted: January 10, 2021











 text in
text in