My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
On-line version ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.11 n.5 Aug./Sep. 2006
ORAL MEDICINE AND PATHOLOGY
Adenoid cystic carcinoma of the dorsum of the tongue: Presentation of a case
Carcinoma adenoideo quístico del dorso de la lengua: Presentación de un caso clínico
Dolores Carrasco Ortiz1, Beatriz Aldape Barrios1
(1) Teachers of Oral Pathology, School of Dentistry. UNAM
ABSTRACT
Adenoid cystic carcinoma is the most frequent malignant neoplasm of minor salivary glands (76.5%); it is clinically characterized by slow growth, and its most frequent localization is the hard palate. Histopathologically it presents three patterns, cribriform, tubular and solid; the solid type is related to a poor prognostic contrary to the cribriform type, which has a better prognosis.
Surgical excision with wide margins is the treatment of choice, if it metastasizes to lymph nodules, post surgical radiotherapy is recommended.
A 19 year-old man presented a recurrent lesion on the dorsum of the tongue previously diagnosed as monomorphic adenoma. In a second biopsy it was diagnosed as adenoid cystic carcinoma. The following immunohistochemical studies were ordered: CALP, CEA, Epithelial Membrane Antigen, Glial Fibrilar Acid Protein, Ki67; all of these studies were positive and with different intensities, corroborating the diagnosis of adenoid cystic carcinoma. The patient had a recurrence after 2 years.
Key words: Adenoid cystic carcinoma, mayor and minor salivary glands, immunehistochemestry.
RESUMEN
El carcinoma adenoideo quístico es la neoplasia maligna de glándulas salivales menores más frecuente (76.5%), se caracteriza clínicamente por ser de crecimiento lento, la localización más frecuente es el paladar duro. Histopatológicamente presenta tres patrones, cribiforme, tubular y sólido; el tipo sólido esta relacionado con un pobre pronóstico a diferencia del tipo cribiforme que tiene un mejor pronóstico. El tratamiento de elección es la excisión quirúrgica con márgenes amplios y cuando existe metástasis a nódulos linfáticos está indicada la radioterapia posquirúrgica.
Se presenta el caso clínico de un hombre de 19 años de edad con recidiva de lesión en dorso de lengua y diagnóstico previo de adenoma monomorfo. En la segunda biopsia se diagnostica como carcinoma adenoideo quístico, por lo que probablemente hubo un error en el diagnóstico original, se decide usar inmunohistoquímica: CALP, CEA, Antígeno Epitelial de Membrana, Proteína Ácido Fibrilar Glial, Ki67, las cuales se observaron positivas en diferentes intensidades, lo que corroboró el diagnóstico de carcinoma adenoideo quístico. El paciente presentó recidiva después de 2 años.
Palabras clave: Carcinoma adenoideo quístico, glándulas salivales mayores y menores, inmunohistoquímica.
Introduction
Adenoid cystic carcinoma is an epithelial malignant neoplasm of salivary glands; which was initially described by Robin and Laboulbene in 1853. (1)
The most frequent localization is the parotid gland (30%), follow by the submandibular region; 40% are found in the minor salivary glands, and almost 1% in the sublingual glands.(2)
The frequency reported in the tongue is 19.8%, with 85% observed at the base of the tongue. (3)
There had been only two cases reported on the dorsum of the tongue. (4)
Clinically it is characterized as a painless slow growing mass o with a propensity to invade peripheral nerves and having a high recurrence rate with metastasis to other organs.
Histopathologically presents three patterns, cribriform, tubular and solid; the most common variant is the cribriform pattern, in which the epithelial cells are arranged in multiple cylindrical spaces, having a pseudo cystic appearance, and many of these pseudo cysts contain a hyaline material.
The tubular type is made up of ducts that can be formed by one or two layers of cells similar to the myoepithelial cells. The solid variant is composed of solid epithelial islands with central areas of necrosis; the cells are small, basophilic and hyperchromatic with a densely granulated nucleus and scarce mitotic figures. (5-6)
The solid type has a poor prognosis contrary to the cribriform type which has a better prognosis. Surgical excision with wide margins is the treatment of choice and, when it metastasizes to the lymph nodes, post surgical radiotherapy is recommended.
The most important prognosis factors include primary lesion size (T), anatomical localization, presence or absence of metastasis (M) at diagnosis time, invasion of the facial nerve and the histopathology grade (G). (6)
Immunohistochemistry studies by Chen and Gnepp in 1988 (7) demonstrated that there were, at least, two cellular populations: luminal cells which express carcino embryonic antigen and membrane epithelial antigen, this indicates its ductal origin; and those non luminal cells that express actin, like actin specified for smooth muscle for myoepithelium characteristics.
In our study other immunohistochemical stains were done, such as S-100 protein, Calponina (myoepithelial cells), glial fibrilar acid protein (GFAP), Ki67 (expressed during G1, S, G2, M phases), which confirm the diagnosis and the malignant biological behavior of the lesion.
Clinical case
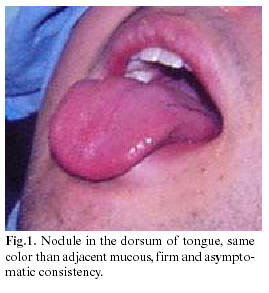
A 19 year-old man, relates having gone to his private dentist three and a half years ago because of a nodule in the dorsum of the tongue, of the same color of the adjacent mucosa, firm consistency, asymptomatic, and one centimeter in diameter. A biopsy was done and the pathologist report renders the diagnosis of monomorphic adenoma type canalicular adenoma.
Three years later, he visited his dentist again because of recurrence of the lesion (Figure 1). The intraoral examination at that time revealed a mass in the dorsum of the tongue at the same place of the first lesion, with light pain to pressure, without cervical lymphadenopathy, laboratory blood studies were requested, which rendered normal results a second biopsy was performed.
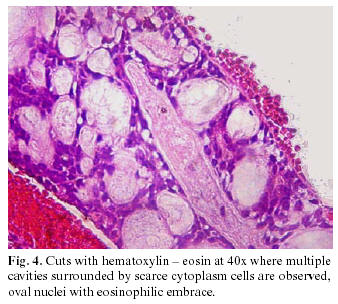
The macroscopic specimen had a firm consistency with an irregular form and surface, brown color and measuring 10 x 6 x 5mm. (Figure 2). The histopathologic study revealed multiple pseudocystic cavities of variable size composed of cubic cells with scarce cytoplasm, oval nuclei with eosinophilic content, surrounded by a band of dense fibrous conjunctive tissue, well vascularized, and moderate diffuse chronic inflammatory infiltration. Aligned cords of cubic cells with hyperchromatic nuclei were seen in the fibrous tissue surrounding the main lesion; in the periphery it presented a perineural infiltration with striated muscular tissue.(Figures 3, 4).
The diagnosis of adenoid cystic carcinoma was established. It was decided to carry out a panel of the following immunohistochemical studies: S-100 protein, actin specified for smooth muscle, CALPONINA, CEA, Membrane Epithelial Antigen, GFAP, and Ki67.
The results of these markers were: Positive CEA in the cytoplasm and in the luminal cells membrane (usually found in striated ducts of minor salivary glands); positive EMA in cytoplasm of intercalated and striated ducts (abundant in the myoepithelial cells around the acinus); positive S-100 in myoepithelial cells, intercalated and striated ducts.
The CALP was positive and therefore the presence of myoepithelial cells is confirmed (Figure 5); Ki-67 stain demonstrated scarce focal areas with approximately 15 to 20% of nuclear stain, and when it is relates to the clinic behavior it indicates that it is a low grade tumor. Once the diagnosis was confirmed, the patient was referred to a cancer center for appropriate treatment and long term follow-up. After 2 years the lesion recurred and then a partial glosectomy was performed and a long term follow-up was indicated.
Discussion
Adenoid cystic carcinoma developing in the dorsum of the tongue is extremely rare; only two reports exist in the literature. (4)
The controversy that this malignant neoplasm presents relates to clinical staging, the histopathological grading and the treatment.
In our case we found that only two out of the three histological patterns prevailed, the classic cribriform type consisting of pseudocystics, that divide the lobule in numerous cylinders giving it the appearance of Swiss cheese or honeycomb; but in the periphery, there were scarce simple cubic cells infiltrating the pseudocapsule. These types of neoplasm composed by two patterns, smaller than 1 cm., have an excellent prognosis when wide surgery is performance.
A good prognosis is influenced by the histopathology type, this relationship has been studied by Batsakis (6), who proposed that the cribriform pattern has a better prognosis. Without solid component it has a 39% survival rate of 15 years; if present in 30% or fewer has 26% survival rate of 15 years and those that are mainly solid have 5% of survival rate of 15 years.
Nascimento (11) and Spiro (6) reported that young patients with short evolution symptoms (1 year) can have a better prognosis. (8)
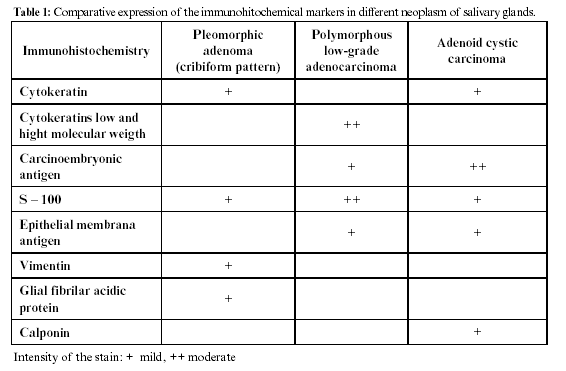
Pleomorphic adenoma with cribriform pattern and polymorphous low grade adenocarcinoma, should be considered in the differential diagnosis since they present similarities in the histological pattern, however, the immunohistochemistry markers for each one of them are decisive in the final diagnosis. (12)
The perineural invasion was not observed in the first biopsy (that is reported in more than 76% of all forms of this neoplasm). Since the lesion was not completely excised, it infiltrated the nervous tissue causing pain in the recurrence, something that was histopathologically demonstrated in the second biopsy with infiltration to the nervous tissue closely related to a high recurrence rate and metastasis. (9-13)
Vrielinck (14) reports that survival rate of 5 years for patients with perineural invasion was significantly inferior (p<0.001) than those that did not have such finding (36.9% vs 93.8%). The evolution between the first clinical manifestations and the presence of symptoms is around 2, 5 to 7 years; our case being in this range after presenting the first symptoms.
The controversial discrepancies of the histological classification of adenoid cystic carcinoma are due to the lack of uniformity in histological criteria to classify its types. The absence of solid areas can be the most important histology characteristic to predict the clinical development of the lesion, as well as an appropriate surgical excision with wide margins. (15)
It has been proposed that the adenoid cystic carcinoma should be divided into two immunohistochemical groups: a group of neoplasm cells with prevalence of ductal formation positive to CEA, EMA, cytokeratin of low and high molecular weight and S- 100.
The other group of neoplasic cells expresses positively for actin of specified smooth muscle and cytokeratin of low molecular weight. (7)
In our case the two cellular lines were expressed positively for CEA and EMA indicating their origin from the ducts cells, and the other cells type was confirmed positively for actin of smooth muscle as it is in cells of myoepithelial origin.
With the histopathological diagnosis of adenoid cystic carcinoma, the referral to a cancer center is done, where all the studies to discard metastasis are carried out; the surgical treatment was partial glosectomy as well as radiotherapy, after two years follow-up there is no recurrence of the lesion.
Acknowledgement: Dr. Douglas Gnepp for his support performance in immunohistochemistry.
![]() Address for correspondence:
Address for correspondence:
Dra. Dolores Carrasco Ortiz
Ixtaccihuatl 11
Col. Condesa
México D.F. 6100
E-mail: patobu1@servidor.unam.mx
Received: 4-06-2004
Accepted: 21-05-2006
References
1. Whear NM, Addy JM. Adenoid cystic carcinoma of the sublingual gland – an unusual presentation. British Journal of Oral and Maxillofacial Surgery 1993; 31:113–6. [ Links ]
2. Ledesma MC, Garcés OM. Salivary gland tumours in a Mexican sample. A retrospective study. Med Oral 2002; 7:324-30. [ Links ]
3. Spiro RJ, Huvos GA. Stage means more than grade in adenoid cystic carcinoma. The American Journal of Surgery 1992; 164:26-30. [ Links ]
4. Ishikawa YU, Ishii TO, Asuwa NO. Adenoid Cystic Carcinoma originated from an anterior lingual minor salivary gland: Inmmunohistochemical and ultrastructural studies and review of the literature. Journal Oral Maxillofac Surg 1997; 55:1460-9. [ Links ]
5. Perzin KH, Gullane P, Clairmont AC. Adenoid cystic carcinomas arising in salivary glands: A correlation of histologic features and clinical course. Cancer 1978;42:265-82. [ Links ]
6. Batsakis JG, Luna MA. Histopathologic grading of salivary gland neoplasms: III. Adenoid cystic carcinomas. Annals of Otology Rhinology and Laryngology 1990: 99; 1007-09. [ Links ]
7. Chen JC, Gnepp DR, Bedrossian CW. Adenoid cystic carcinoma of the salivary glands: An inmunohistochemical analysis. Oral Surgery Oral Medicine Oral Pathology 1988; 65:316-26. [ Links ]
8. Araujo VC, Sousa SO, Carvalho YR. Application of immunohistochemistry to the diagnosis of salivary gland tumors. Virchows Arch 2000; 1:58-68. [ Links ]
9. Gormley WB, Sekhar LN, Wright DC, Olding M. Management and long-term outcome of adenoid cystic carcinoma with intracranial extension: neurosurgical perspective. Neurosurgery 1996; 38:1105-13. [ Links ]
10. Andersen LJ. Therlildsen MH, OckelmannHH, Bentzen JD, Schiodt T, Hansen HS. Malignant epithelial tumours in the minor salivary glands, the submandibular salivary gland and the sublingual gland. Cancer 1991; 69:615-9. [ Links ]
11. Nascimento AG, Amaral A, Prado L, Kligerman J, Silvera R. Adenoid cystic carcinoma of salivary glands. Cancer 1986; 57:312-9. [ Links ]
12. Spiro RH, Thaler HT, Hicks WF. The importance of clinical staging minor salivary gland carcinoma. American Journal Surgery 1991; 162:330-6. [ Links ]
13. Cerulli G, Renzi G, Perugini M, Becelli R. Differential diagnosis between adenoid cystic carcinoma and pleomorphic adenoma of the minor salivary glands of palate. Journal of Craniofacial Surgery 2004: 6:1056-60. [ Links ]
14. Vrielinck LJ. The significance of perineural spread in adenoid cystic carcinoma de la major an minor salivary glands. International Journal of Oral and Maxillofacial Surgery 1988; 17: 190-3. [ Links ]
15. Waldron CA, Gnepp DR. Tumours of the intraoral minor salivary glands: A demographic and histologic study of 426 cases. Oral Surgery Oral Medicine Oral Phathology 1988; 66:323-33. [ Links ]











 text in
text in