Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.12 no.4 ago. 2007
Localized leishmaniasis of the oral mucosa. A report of three cases
José Antonio García de Marcos1, Alicia Dean Ferrer2, Francisco Alamillos Granados3, Juan José Ruiz Masera4, Begoña Cortés Rodríguez5, Alfredo Vidal Jiménez6, Ana García Lainez7, Aquiles Lozano Rodríguez-Mancheño8
(1) Consultant. Department of Oral and Maxillofacial Surgery. Albacete University Hospital. Albacete
(2) Head of Section. Department of Oral and Maxillofacial Surgery. University Hospital "Reina Sofía". Cordoba.
Fellow of the European Board of Oral and Maxillofacial Surgery. Associated Professor. School of Medicine. Córdoba University
(3) Consultant. Department of Oral and Maxillofacial Surgery. University Hospital "Reina Sofía". Cordoba, Spain.
Fellow of the European Board of Oral and Maxillofacial Surgery. Associated Professor. School of Medicine. Córdoba University
(4) Consultant. Department of Oral and Maxillofacial Surgery. University Hospital "Reina Sofía". Cordoba
(5) Consultant. Department of Internal Medicine. Sierra del Segura Hospital. Jaen
(6) Resident. Department of Pathology. University Hospital "Reina Sofía". Cordoba
(7) Resident. Department of Oral and Maxillofacial Surgery. University Hospital "Reina Sofía". Cordoba
(8) Resident. Department of Internal Medicine. University Hospital "Reina Sofía". Cordoba, Spain
ABSTRACT
The term leishmaniasis comprises a group of diseases caused by different species of a protozoon called Leishmania. Leishmaniasis is found worldwide, and is considered to be endemic in 88 countries. There are three main clinical forms of leishmaniasis: visceral leishmaniasis, cutaneous leishmaniasis and mucocutaneous leishmaniasis. Exclusive involvement of the mucosa is very rare. We present a series of three cases of mucosal leishmaniasis located in the oral cavity. The fact that all three cases were recorded in Spain (an area where L. infantum is endemic), suggests that the latter was the causal agent. The only manifestation of leishmaniasis disease in the described cases was the appearance of an oral lesion. Treatment was provided in the form of meglumine antimoniate in two patients, with a favorable response. One of the patients left the hospital after diagnosis, without receiving treatment, and the subsequent course is not known. A review is made of the literature on the subject.
Key words: Mucosal leishmaniasis, leishmania infantum, mediterranean leishmaniasis.
RESUMEN
El término leishmaniasis comprende un grupo de enfermedades causadas por diferentes especies de un protozoo llamado Leishmania. La leishmaniasis se produce en todo el mundo, considerándose endémica en 88 países. Existen tres formas clínicas principales de leishmaniasis: leishmaniasis visceral, leishmaniasis cutánea y leishmaniasis mucocutánea. La afectación de la mucosa, de manera exclusiva, por la Leishmania es muy rara. Presentamos una serie de tres casos de leishmaniasis mucosa localizados en la cavidad oral. El hecho de que todos los casos se produjeran en España, área endémica de L infantum, nos hace presuponer que éste fue el agente causal. La única manifestación de enfermedad de leishmaniasis en los casos descritos, fue la aparición de una lesión oral. Se administró tratamiento con antimoniato de meglumina en dos de ellos, respondiendo favorablemente. Uno de los pacientes abandonó el hospital tras el diagnóstico sin recibir tratamiento y se desconoce la evolución. Realizamos también una revisión de la literatura.
Palabras clave: Leishmaniasis mucosa, leishmania infantum, leishmaniasis mediterránea.
Introduction
Leishmaniasis, caused by a heterogeneous group of protozoan parasites belonging to the genus Leishmania, produces a number of clinical syndromes (1-7). Rodents and canines (both wild and domestic) constitute the natural reservoir of Leishmania, which is transmitted from animals to humans, and occasionally from one human to another, by means of the female mosquito (Phlebotomus spp. in the Old World and Lutzomyia spp. in the Americas)(2,3,5,6,8,9). The mosquito sucks the blood of humans or mammals, containing the protozoon in its amastigote form (lacking a flagellum). Once within the intestine of the mosquito, the parasite transforms into a promastigote (equipped with a flagellum), and reproduces as such (5,8,10). From the intestine of the mosquito, the protozoon then migrates to the esophagus, where it is expelled into the skin of the host when the insect bites - followed by invasion of the bloodstream and different tissues (5,8,10). The promastigotes are phagocytosed by the macrophages of the host reticuloendothelial system, where they lose the flagellum and once again transform into amastigotes. When the infected cells are destroyed, the parasite infects new cells and thus spreads throughout the body (5,8,10).
Leishmaniasis is found throughout the world, except in Australia and the Antarctic, and is considered to be endemic in 88 countries (1,5,10). Although the disease manifests mainly in such endemic areas, people visiting such zones can become infected after less than a single week of exposure (11). It is estimated that 350 million people are at risk of contracting the illness, and its prevalence is 12 million infected individuals worldwide - with a global incidence of 1.5-2 million new cases per year (1,2,5,8,10). Leishmaniasis is responsible for approximately 70,000 to 80,000 deaths a year (8,10).
Over 20 different species of Leishmania have been described (3,5,8). There are three clinical forms of leishmaniasis: visceral leishmaniasis (VL), cutaneous leishmaniasis (CL), and mucocutaneous leishmaniasis (MCL)(6,10). Each of them is generally associated with certain species and concrete geographical settings. Thus, CL in the Americas is caused by L. mexicana, L. amazonensis, L. braziliensis, L. panamensis, L. peruviana and L. guyanensis, while in the Old World it is caused by L. tropica, L. major, L. aethiopica and L. infantum. In turn, VL is caused by L. donovani in India, Asia and Africa, while in the Mediterranean, North Africa, Central and Southeast Asia and South America it is caused by L. infantum or L. chagasi. MCL, which is the least frequent clinical form of the disease, is caused by L. braziliensis in the Americas, while and in the Old World L. aethiopica is responsible (1-4,8-10). However, it is increasingly clear that species commonly associated with VL can produce skin or mucosal lesions, and vice versa.
Exclusive involvement of the mucosa is very rare. We present a series of three cases of mucosal leishmaniasis located in the oral cavity.
Clinical cases
Table 1 describes the characteristics of the three patients presented in this study (table 1).
Case 1. A 70-year-old male was referred to the maxillofacial surgery unit for evaluation of an ulcerated and painful lesion measuring 2 cm in diameter, located in the anterior region of the floor of the mouth, around the outlet of the right Whartons duct. The lesion was indurated and presented irregular margins. As personal antecedents, the patient had type 2 diabetes mellitus subjected to treatment with oral antidiabetics, and interstitial desquamative pneumopathy treated with low-dose corticoids. He had undergone prostatectomy, and presented a history of deep venous thrombosis and relapsing orchiepididymitis. The patient was HIV-negative. A biopsy of the lesion was obtained under local anesthesia. The histological study revealed epithelium with a chronic inflammatory infiltrate mainly composed of plasma cells and histiocytes, with a cytoplasm invaded by Leishmania parasites. Abdominal ultrasound was subsequently performed, revealing no alterations, and a peripheral mononuclear cell culture proved negative. A bone marrow biopsy showed no alterations. Both the laboratory test and hematometric findings proved normal. Immunofluorescence showed the Leishmania IgG antibody titer to be 1:320.
The condition was classified as mucosal leishmaniasis, and treatment was started with meglumine antimoniate (Glucantime®) at a dose of 20 mg/kg/day, during 28 days - with gradual disappearance of the lesion. No adverse effects of treatment were observed. After 10 years of follow-up, the patient showed no signs of relapse.
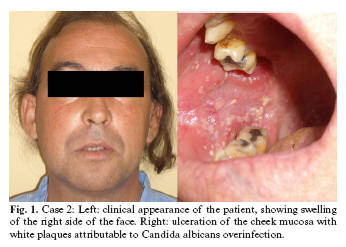
Case 2. A 41-year-old male presented with a painful ulcer measuring 3 cm in diameter on the right cheek mucosa (Fig. 1). The personal history included HIV infection for the previous 25 years, with criteria of AIDS disease (stage C3). No antiretroviral therapy was being provided upon personal request by the patient, who was an ex-intravenous drug abuser with hepatitis C infection and tuberculosis two years before. The histopathological study of the biopsy revealed leishmaniasis with superficial Candida albicans coinfection. The lymphocyte subpopulation counts were as follows: CD4: 170 cells/mm3; CD8: 865 cells/mm3; CD4/CD8: 0.20. The HIV viral load was 160,000 copies/ml. Abdominal ultrasound and a bone marrow aspirate proved normal. The condition was classified as mucosal leishmaniasis, and treatment was started with antiretrovirals (lamivudine 150 mg / zidovudine 300 mg (Combivir®) and nevirapine 200 mg (Viramune®)) and meglumine antimoniate (Glucantime®) 20 mg/kg/day. One month after treatment, the patient developed daily vomiting, asthenia, anorexia, febrile sensation and pruritus. Laboratory testing in turn established liver enzyme elevations - the diagnosis being liver toxicity secondary to the treatment provided. Meglumine antimoniate was therefore withdrawn, followed by the antiretroviral therapy - resulting in clinical improvement and normalization of the liver enzymes. After this period of time, comprising one month of treatment with meglumine antimoniate, the patient responded favorably, with gradual disappearance of the lesion on the cheek mucosa.
One month after the end of treatment with meglumine antimoniate, secondary prophylaxis was started with aromatic diamidines (pentamidine isethionate) every 15 days (4 mg/kg b.w.), and maintained on occasion of the last control. One year after the end on treatment with meglumine antimoniate, no evidence of relapse was observed.
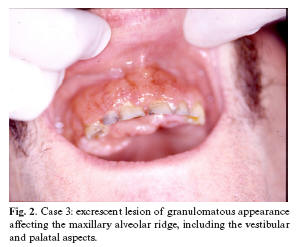
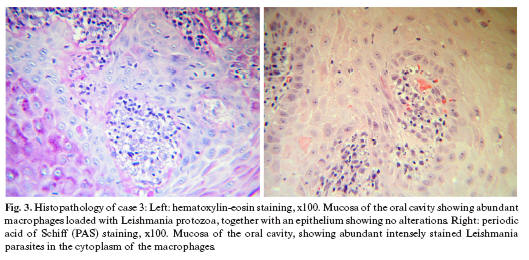
Case 3. A 50-year-old male presented with a two-month lesion located in the maxillary alveolar ridge region extending from tooth 17 to 22, and affecting both the vestibular aspect and the palatal surface - with a maximum diameter of 5 cm. The lesion was excrescent, granulomatous, hemorrhagic and painful (Fig. 2). The patient presented no other clinical manifestations. The personal history comprised HIV infection, hemophilia and surgery for a duodenal ulcer. The histological study of a biopsy of the oral lesion indicated leishmaniasis (Fig. 3). The patient left the hospital after diagnosis, without receiving treatment, and the subsequent course is not known.
Discussion
Mucosal involvement due to New World Leishmania species (L. braziliensis) is observed in 1-10% of cases, generally developing 1-5 years after the healing of cutaneous leishmaniasis, though in some cases mucosal and skin lesions can coincide (8,9). Approximately 90% of these patients present a prior cutaneous scar (8,9). Occasionally, the mucosal lesions may also appear in patients with no previous skin lesions (9). Mucosal leishmaniasis usually starts as erythema and ulceration, generally in the nasal fossa, and evolves towards perforation of the septum and a destructive inflammatory lesion. Mucosal disease caused by L. braziliensis occasionally has been described outside Latin America, and can be acquired by traveling persons (8).
Infection due to L. infantum is found in Southern Europe, North Africa, the Middle East, Pakistan and China. In the Mediterranean, the parasite can cause VL, CL, as well as localized mucosal leishmaniasis. Aliaga et al. reviewed localized mucosal leishmaniasis caused by L. infantum in a total of 31 cases (12).
The authors consider a patient to have localized mucosal leishmaniasis secondary to L. infantum when the following criteria are met: 1) acquisition of the disease in areas that are endemic for L. infantum; 2) identification of the parasite in the oral mucosa biopsy (both tissue staining and culture); 3) absence of the parasite (visualization or culture) in bone marrow aspirate (or in liver or spleen aspirate); and 4) no clinical evidence of visceral or cutaneous leishmaniasis (12). Our first two patients meet all the criteria for localized mucosal leishmaniasis due to L. infantum. The third case would have required a bone marrow aspirate, for example, to discard the presence of Leishmania in other locations. Of the cases reviewed by Aliaga et al. (12), 90% corresponded to males. The mean age was 48 ± 14 years. Fourteen cases were documented in Spain (45.16%), 9 in Italy (29%), 4 in France (12.9%), 2 in England (6.45%), and one case each in Morocco and the United States (3.22%). Seven of the patients (22.58%) showed HIV coinfection (12). All three of our patients were men, with a mean age of 53.6 years. All three were Spaniards living in Spain, and none had recently traveled abroad. Two of them (66.6%) had HIV coinfection, and the third was receiving immune suppressive therapy in the form of corticoids.
In the review published by Aliaga et al. (12), lesions are described in the mucosa of the nose, cheek, lips, soft palate, hard palate, tongue, tonsils and larynx. The lesions are generally red or purple in color and manifest as a nodule, tumor, polypoid lesion or granular inflammation. Ulcerations can be found on the tongue, tonsils, lips, palate and vocal cords. The lesions were painless in all patients except two (12). In our series the lesions were located on the cheek mucosa, the floor of the mouth and maxillary mucosa. In two cases the lesions manifested as ulcers, while the third patient presented a granulomatous tumefaction. The lesions were painful in all three cases.
Mediterranean mucosal leishmaniasis shows numerous differences with respect to mucosal leishmaniasis caused by L. braziliensis. In the latter presentation of the disease, the nasal cavity is affected in 90% of cases, while in mucosal leishmaniasis in Mediterranean countries such lesions are found in only 16% of cases, approximately. Moreover, Mediterranean mucosal leishmaniasis has a better prognosis than mucosal leishmaniasis in South America. The number of parasites found in the lesions of mucosal leishmaniasis caused by L. braziliensis is limited, while the parasite burden found in lesions of Mediterranean mucosal leishmaniasis is important (12).
The incidence of leishmaniasis as an opportunistic disease has increased in recent years due to the growing number of patients with immune depression secondary to chronic illnesses, neoplasms, transplants, immunosuppressive treatments, and HIV infection (1-3,5,6). In patients with HIV, Leishmania infection is not very common, though it is believed that 50% of adult patients with visceral leishmaniasis are HIV-positive (3). Although the transmission of leishmaniasis in patients coinfected with HIV most often occurs via the mosquito vector, there have been reports of alternative routes of transmission, likewise shared by HIV, such as parenteral inoculation (intravenous drug abusers who share needles, blood transfusions, laboratory accidents, etc.), sexual transmission, organ transplantation, and vertical (trans-placental) transmission (1-5,8,9). The World Health Organization (WHO) estimates that 2-3% of AIDS patients in Southeast Europe have developed this opportunistic infection (2). Leishmaniasis can manifest in any stage of HIV infection, though the diagnosis typically coincides with periods of marked host immune depression. Some authors consider that this may be due to the lymphopenia induced by leishmaniasis itself (2,3).
The differential diagnosis of oral mucosal leishmaniasis must be established with fungal infections (blastomycosis), syphilis, tuberculosis, leprosy, sarcoidosis, midline granuloma, Wegeners disease and neoplasms, among other disorders (13,14).
The treatment of choice in all clinical forms of leishmaniasis is based on the administration of pentavalent antimonial drugs such as meglumine antimoniate (Glucantime®) and sodium stibogluconate (Pentostam®). The former drug is more widely used, preferentially via the intramuscular route - with intravenous dosing as secondary alternative (3,5,9,12,15). The meglumine antimoniate dosage recommended by the United States Centers for Disease Control (CDC) and the WHO is 20 mg/kg b.w./day, via the intramuscular or intravenous route, to a maximum of 850 mg/day (to avoid side effects), for at least 20 days - with extension during at least two weeks beyond apparent parasitological healing (2,9,15). Mediterranean mucosal leishmaniasis should be treated for 28 days, though shorter therapies may also be appropriate (12). The possible adverse effects of these drugs are: myalgia, joint pain, anorexia, nausea, vomiting, headache, electrocardiographic changes (T-wave inversion, prolongation of the QT interval), hypertransaminemia, chemical pancreatitis, thrombocytopenia and neutropenia (5,9,15,16). As an alternative to such treatment, patients can be prescribed amphotericin B, pentamidine, rifampicin or ketoconazole (2,9,12,15,17). Other drugs that have been used for treatment are: phenothiazines, paromomycin, allopurinol, itraconazole, cyclosporine, miltefosin, dapsone, aminosidine and interferon (2,9,12,15,16). Other management options include surgery, topical treatments, immunotherapy, and infrared heat (2,6,9,12). In patients with Leishmania and HIV coinfection, the same management protocol is applied, though some authors recommend life-long prophylaxis with aromatic diamidines (Pentamidina®) in aerosol, to avoid relapses (5). Our first two cases were treated with meglumine antimoniate (Glucantime®) 20 mg/kg/day, during one month. In the second case, and following initial treatment, secondary prophylaxis was provided in the form of aromatic diamidines (Pentamidina®) every 15 days (4 mg/kg b.w.). The third patient received no treatment, since he left the hospital following diagnosis of the disease.
Our first two patients were seen to be free of disease after one and 10 years of follow-up, respectively.
Conclusions
The incidence of leishmaniasis as an opportunistic disease has increased, due to the growing number of immune depressed patients. As a result, leishmaniasis of the oral mucosa must be considered in the differential diagnosis of oral lesions, in those geographical settings where the parasite is endemic.
References
1. Sinha PK, Pandey K, Bhattacharya SK. Diagnosis & management of leishmania/HIV co-infection. Indian J Med Res 2005;121:407-14. [ Links ]
2. Vázquez-Piñeiro T, Fernández Álvarez JM, Gonzalo Lafuente JC, Cano J, Gimeno M, Berenguer J. Visceral leishmaniasis. A lingual presentation in a patient with HIV infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;86:179-82. [ Links ]
3. Milián MA, Bagán JV, Jiménez Y, Pérez A, Scully C. Oral leishmaniasis in HIV-positive patient. Report of a case involving the palate. Oral Dis 2002;8:59-61. [ Links ]
4. Abbas K, el Toum IA, el Hassan AM. Oral leishmaniasis associated with kala-azar. A case report. Oral Surg Oral Med Oral Pathol 1992;73:583-4. [ Links ]
5. Alvar Ezquerra J. Leishmaniasis. En: Farreras P, Rozman C,eds. Medicina Interna. Madrid: Harcourt Brace; 1998.p.2444-7. [ Links ]
6. Habibzadeh F, Sajedianfard J, Yadollahie M. Isolated lingual leishmaniasis. J Postgrad Med 2005;51:218-9. [ Links ]
7. Milian A, Bagán JV, Carbonell E, Lloria E, Cardona F. Leishmaniasis: report of a case with oral lesions. Med Oral 1996;1:114-8. [ Links ]
8. Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet 2005;366:1561-77. [ Links ]
9. Costa JW Jr, Milner DA Jr, Maguire JH. Mucocutaneous leishmaniasis in US citizen. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;96:573-7. [ Links ]
10. Hsia R, Wang N, Halpern J. Leishmaniasis. 2005. "www.emedicine.com/emerg/topic296.htm" [ Links ]
11. Scope A, Trau H, Bakon M, Yarom N, Nasereddin A, Schwartz E. Imported mucosal leishmaniasis in a traveler. Clin Infect Dis 2003;37:E83-7. [ Links ]
12. Aliaga L, Cobo F, Mediavilla JD, Bravo J, Osuna A, Amador JM, et al. Localized mucosal Leishmaniasis due to Leishmania (Leishmania) infantum: clinical and microbiologic findings in 31 patients. Medicine 2003;82:147-58. [ Links ]
13. Sastre Pérez J, Muñoz Guerra MF, Naval Gías L, Martín Granizo R, Elices de Apellaniz M, Díaz González FJ. Leishmaniasis oral: A propósito de un caso y revisión de la literatura. Rev Esp Cirug Oral y Maxilofac 1999; 21:338-42. [ Links ]
14. Chimenos-Kustner E, Pascual-Cruz M, Pinol-Dansis C, Vinals-Iglesias H, Rodriguez-de Rivera-Campillo ME, Lopez-Lopez J. Lepromatous leprosy: A review and case report. Med Oral Patol Oral Cir Bucal 2006;11:E474-9. [ Links ]
15. Berman JD. Treatment of New World cutaneous and mucosal Leishmaniases. Clin Dermatol 1996;14:519-22. [ Links ]
16. Sundar S, Jha TK, Thakur CP, Engel J, Sindermann H, Fischer C, et al. Oral miltefosine for indian visceral leishmaniasis. N Engl J Med 2002;347:1739-46. [ Links ]
17. Van Damme PA, Keuter M, Van Assen S, De Wilde PCM, Beckers PJA. Clinical picture. A rare case of oral leishmaniasis. Lancet Infect Dis 2004;4:53. [ Links ]
![]() Correspondence:
Correspondence:
Dr. J.A. García de Marcos.
C/ Antonio Acuña. Nº10.5ºAizq.
28009. Madrid, España.
E-mail: pepio2@hotmail.com
Received: 31-07-2006
Accepted: 26-01-2007