Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.12 no.6 oct. 2007
Secretor status and ABH antigens expression in patients with oral lesions
Carlos Campi1, Livia Escovich1, Vanina Valdés2, Silvia García Borrás2, Liliana Racca2, Amelia Racca2, Carlos Cotorruelo2, Claudia Biondi2
(1) Cátedra de Estomatología. Facultad de Odontología. Universidad Nacional de Rosario. Córdoba. Rosario
(2) Laboratorio de Inmunohematología Histocompatibilidad e Inmunogenética. Departamento de Bioquímica Clínica. Facultad de Ciencias Bioquímicas y Farmacéuticas. Universidad Nacional de Rosario. Suipacha. Rosario. Argentina
ABSTRACT
Objectives: The aim of this work was to investigate the secretor status of patients with oral pre-cancerous and cancerous lesions and ABH antigens expression in fixed tissue sections of these patients.
Study design: To reveal A, B and H antigens in tissue sections of patients with precancerous and cancerous oral lesions (n= 54) we used a modified specific red cell adherence technique (SRCA-test). Normal endothelial cells expressed ABH antigens, the presence of indicator erythrocytes at the lumen of the blood vessels served as a built in positive control. The test results were graded from negative adherence to very strongly positive adherence. Negative adherence was defined as a complete absence of adhered indicator erythrocytes. A strongly positive reaction was defined as a sheet of indicator erythrocytes adhered to the epithelia cells.
Results: In 31 of the 54 samples analyzed the test showed slightly positive results on atypical areas, and there was a complete antigen deletion in areas histologically affected by neoplasia. Sixteen samples showed a total absence of ABH antigens in both histologically normal and pathological areas. As a working hypothesis, we propose that areas of SRCA-test negative epithelium are closely related to invasive carcinomas and may be their precursor lesions. Further it is suggested that areas of blood group isoantigen negative epithelium showing atypia, or in some instances near normal histology, may give rise to relatively low grade carcinomas.
Conclusions: Considering these results we suggest the use of this method to monitor probable preneoplastic lesions in risk population, specially in those with no secretor status.
Key words: Secretor Status, ABH antigens, cancer.
Introduction
Histo-blood group ABH (O) antigens are the major allogeneic antigens in humans and they are widely distributed in human tissues. Their presence not only is limited in blood cells but also is found in various epithelial cells (1, 2). Since most human cancers originate from epithelial cells, changes in blood group antigens are an important topic in human tumor immunology. Glycolipids constitute an essential part of blood group antigens present at the cell surface membranes. In human tumors, blood group antigens change in the same general direction as other glycosphingolipids do in tumors (3, 4).
Tumor progression is often associated with altered glycosylation of the cell-surface proteins and lipids (5). The peripheral part of these cell-surface glycoconjugates often carries carbohydrate structures related to the ABO and Lewis blood-group antigens. The expression of histo-blood-group antigens in normal human tissues is dependent on the type of differentiation of the epithelium. In most human carcinomas, including oral carcinoma, a significant event is decreased expression of histo-blood-group antigens A and B (6). The mechanisms of aberrant expression of blood-group antigens are not clear in all cases. (5-9). It has been demonstrated in a number of earlier studies on the etiology and pathogenesis of certain diseases that the patients secretor status (ABO (H) blood group antigens) may probably be a factor influencing the development of systemic oral diseases (10). This likelihood has prompted the present study, to examine the differences in the saliva secretor status by comparing patients with oral pre-cancerous and cancerous lesions on the one hand, and the healthy population on the other; in relation to the ABH antigens expression in fixed tissue sections of these patients.
Materials and methods
In total 108 subjects were examined, half of whom suffered from oral pre-cancerous and cancerous lesions, while the other half were the healthy control group. All were subjected to clinical oral examinations and standard evaluation tests in order to establish the secretor status of their saliva (agglutination inhibition technique) (11). In the group of patients with oral pre-cancerous and cancerous lesions (experimental group), a pathohistological examination of the oral mucosa was performed.
Inhibition test for Secretor Status: (11)
2 or 3 mL. of saliva were collected into wide mouthed tubes. In order to eliminate the mucine protein they were treated with thermal shocks. They were centrifuged and the supernatant were transferred to a clean test tube and placed in boiling water bath for 10 minutes to inactivate salivary enzymes.
To 1 drop of appropriately diluted blood grouping reagent (anti-A, anti-B or ulex europeaus) we added 1 drop of patients saliva. We incubated 10 minutes at room temperature and then we added 2 drops of 2% to 5% saline suspension of washed indicator red cells. Then, the tube was incubated 30 minutes and centrifuged in order to inspect cell button macroscopically for agglutination.
Agglutination of indicator cells by antibody in tubes containing saliva indicates that the saliva does not contain the corresponding antigen (non secretor status, se). Failure of known antibody to agglutinate indicator cells after incubation with saliva indicates that the saliva contains the corresponding antigen (secretor status, se).
Specific red cell adherence test (12)
Specific red cell adherence test was performed on paraffin embedded sections to detect the intensity of isoantigens A, B and H (O) on the epithelial cell surface by a three layer sandwich technique.
Reagents Used
-. Commercially available antisera, Anti A, Anti B, and Anti AB, from span diagnostic limited and lectin ulex europeaus (Anti H).
-. Tris Buffer saline 0.05 M with pH 7.4.
-. 2-5% Red Blood Cells suspension.
Procedure in Brief
Slides of 4-5 micron section were deparaffinized and brought to water, immersed in tris buffered saline 0.05 M (pH 7.4) for 30 minutes, covered with isologous antisera according to patients blood group and incubated for one hour for A, B and O group in a most chamber at room temperature. The slides were then dipped in tris buffered saline for three changes with occasional strings to remove the unreacted antisera. Few drops of 2-5% isologous indicator RBCs suspension were added to the sections and incubated for 20 minutes in group A or B and one hour for group O. The slides were inverted over a support in a petridish containing tris buffered saline such that the undersurface of the slide just touched the solution and kept for five minutes to settle down unreacted RBCs. The slides were observed under low power and photographed immediately.
Controls: Normal tissues containing blood group antigens, endothelium of blood vessels and RBCs acted as inbuilt positive controls and adipose tissues acted as inbuilt negative controls.
Interpretation:
In the present study the isoantigenicity of epithelium was graded according to degree of adherence of indicator RBCs as strongly positive adherence (++++) to negative adherence (-), Levels intermediate were determined as 25% of adherence +, 50% of adherence ++ and 75% of adherence +++.
Statistical Analysis
The categorical data were examined with a Χ2 test, and the ORs were estimated using an unconditional logistic model.
Results
The 79.5 % of the healthy individuals studied posses the Se gene that governs the secretion of water-soluble ABH antigens into saliva (secretor status). These secreted antigens can be demonstrated in saliva by agglutination inhibition tests with ABH antisera. The 51% (n=16) of the patients with oral pre-cancerous and cancerous lesions was non secretors (se), RO = 2.44; IC 95% (0.7836 ; 7.5534) (p= 0.1196) in contrast with the healthy population (Table 1), We observed a marginal association between secretor status (Se) and these lesions.
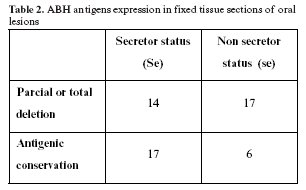
The immunoadherence reaction to tissue sections using antibodies and red blood cells showed a loss of A, B or H antigens related to the stage of tumor (Table 2). We found a higher intensity of oral disease in the non secretor group (se), RO = 3.44; IC95% (1.0682; 11.0729) (p= 0.0346), and the occurrence of epithelial dysplasia was found exclusively in these group.
A loss of ABH reactivity within the most invasive sites of the tumors correlated significantly with the stage of tumor development and histological grade of malignancy.
In the sections tissue studied the endothelium of blood vessels was reactive with the erythrocytes (positive control) and adipose tissues did not react with the red blood cells (negative controls).
Discussion
The membrane, which defines the extent of the cell, is not only a physical boundary but also has many specific functions, among which is the capacity to react with other cells and the intracellular matrix (13, 14). Carbohydrates are structures found on the cell surface bound to either lipid or protein embedded in the membrane. Changes in the carbohydrate structure of these cell-surface glycolipids and glycoproteins have been demonstrated during development, during cell maturation in adult tissue, and in relationship to malignant development (9,15,16,17)
Most studies concerning the tissue localization of the histo-blood-group antigens have shown that the antigens in the tissues correspond to the erythrocyte blood group, but that the tissue expression is dependent on the secretor status of the individual (14). Furthermore, the expression of histo-blood-group antigens in normal human tissues is dependent on the type of differentiation of the epithelium and the degree of maturation of the single cell within the epithelium. In stratified epithelium, the expression of histo-blood-group antigens depends on the state of cellular differentiation (maturation), and there is a sequential elongation of the terminal carbohydrate chain during the life span of the cell. Basal cells express short carbohydrate chains that are A/B precursors, whereas A, B or H antigens may be seen in the spinous cell layer.
The results obtained have demonstrated that the large majority of the people examined in the healthy group were Se and there were significant difference between secretors and non-secretors in the experimental group. We also found a higher intensity of oral disease in the se group, and the occurrence of epithelial dysplasia was most found in these group.
The studies of patients with pre malignant and malignant oral lesions in which non-secretor status predominates, appear to be an associated risk marker for the development for oral cancer.
We also observed a reduction or complete deletion of A, B or H antigen expression in sections of tissues of patients se with oral pre carcinomas or carcinomas. Disappearance of the antigens is ascribed to the absence of A or B transferase gene expression. These findings are consistent with several studies showing that loss of A and B antigen expression is associated with increased tumourigenecity in syngeneic animals.
In summary, our results indicate that at the same time as the morphological changes that occur during the process of oral carcinogenesis, another series of events occurs. Further follow-up studies are required to clarify the role of predictive markers of risk in precursor lesions of oral cancer.
References
1. Mollison PI, Engelfriet CP, Contreras P. Blood Transfusion in Clinical Medicine. 9th Ed. Oxford: Blackwell ScientificPublications; 1993. [ Links ]
2. Salmon C. Les Groupes sanguins ou I´ecriture des Genes. Paris: Masson; 1997. [ Links ]
3. Clausen H, Bennett EP, Grunnet N. Molecular genetics of ABO histo-blood groups. Transfus Clin Biol. 1994;1(2):79-89. [ Links ]
4. Chimenos-Kustner E, Font-Costa I, Lopez-Lopez J. Oral cancer risk and molecular markers. Med Oral Patol Oral Cir Bucal. 2004 Nov-Dec;9(5):381-4; 377-80. [ Links ]
5. Santos-Garcia A, Abad-Hernandez MM, Fonseca-Sanchez E, Julian-Gonzalez R, Galindo-Villardon P, Cruz-Hernandez JJ, et al. E-cadherin, laminin and collagen IV expression in the evolution from dysplasia to oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2006 Mar 1;11(2):E100-5. [ Links ]
6. Le Pendu J, Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Clement M. ABH and Lewis histo-blood group antigens in cancer. APMIS. 2001 Jan;109(1):9-31. [ Links ]
7. Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochim Biophys Acta. 1999 Dec 6;1473(1):247-66. [ Links ]
8. Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci U S A. 2002 Aug 6;99(16):10231-3. Epub 2002 Jul 30. [ Links ]
9. Gao S, Worm J, Guldberg P, Eiberg H, Krogdahl A, Liu CJ, et al. Genetic and epigenetic alterations of the blood group ABO gene in oral squamous cell carcinoma. Int J Cancer. 2004 Mar 20;109(2):230-7. [ Links ]
10. Mandel U, Orntoft TF, Holmes EH, Sorensen H, Clausen H, Hakomori S, et al. Lewis blood group antigens in salivary glands and stratified epithelium: lack of regulation of Lewis antigen expression in ductal and buccal mucosal lining epithelia. Vox Sang. 1991;61(3):205-14. [ Links ]
11. Walkers J. American Association of Blood Banks. Manual técnico. 13th ed. Buenos Aires: Asociación Argentina de Hemoterapia e Inmunohematología; 2002. [ Links ]
12. Strauchen JA, Bergman SM, Hanson TA. Expression of A and B tissue isoantigens in benign and malignant lesions of the breast. Cancer. 1980 Apr 15;45(8):2149-55. [ Links ]
13. Ebnet K, Vestweber D. Molecular mechanisms that control leukocyte extravasation: the selectins and the chemokines. Histochem Cell Biol. 1999 Jul;112(1):1-23. [ Links ]
14. Ravn V, Dabelsteen E. Tissue distribution of histo-blood group antigens. APMIS. 2000 Jan;108(1):1-28. [ Links ]
15. Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochim Biophys Acta. 1999 Dec 6;1473(1):247-66. [ Links ]
16. Hakomori S. Structure, organization, and function of glycosphingolipids in membrane. Curr Opin Hematol. 2003 Jan;10(1):16-24. [ Links ]
17. Santos-Garcia A, Abad-Hernandez MM, Fonseca-Sanchez E, Cruz-Hernandez JJ, Bullon-Sopelana A. Proteic expression of p53 and cellular proliferation in oral leukoplakias. Med Oral Patol Oral Cir Bucal. 2005 Jan-Feb;10(1):5-8; 1-5. [ Links ]
![]() Correspondence:
Correspondence:
Dr. Claudia Biondi
Laboratorio de Inmunohematología Histocompatibilidad e Inmunogenética.
Departamento de Bioquímica Clínica.
Facultad de Ciencias Bioquímicas y Farmacéuticas.
Universidad Nacional de Rosario.
Suipacha 531. 2000 Rosario. Argentina.
Email: cbiondi@fbioyf.unr.edu.ar
Received: 18-08-2006
Accepted: 30-04-2007