Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.12 no.6 oct. 2007
High resolution image in bone biology I. Review of the literature
Jorge Cano1, Julián Campo1, Juan José Vaquero2, Jose María Martínez3, Antonio Bascones4
(1) Lecturer, School of Dentistry, UCM
(2) Researcher, Image Lab, Medicine and Experimental Surgery, Gregorio Marañón General University Hospital
(3) Senior lecturer, School of Dentistry, UCM
(4) Professor, School of Dentistry, UCM. Madrid
ABSTRACT
Bone microstructure has usually been assessed by obtaining samples invasively and analyzing them with conventional histomorphometric methods. Improvements in high-resolution image acquisition systems have enabled non-invasive assessment of bone morphology and a more precise 3-D evaluation by means of "virtual biopsies", permitting bone assessment in regeneration or remodeling processes. This review describes the characteristics and limitations of bone assessment using different high-resolution image systems (synchrotron-radiation computed tomography, micro-computed tomography, acoustic scanning microscope; micro-magnetic resonance imaging). Morphometric variables that can be obtained from these images are reported and compared with conventional histomorphometric variables.
Key words: High-resolution image, bone biology, virtual biopsy.
RESUMEN
La valoración de la microestructura ósea se ha realizado habitualmente mediante la obtención invasiva de muestras y el procesado y evaluación de las mismas con métodos convencionales histomorfometricos. La mejora de los sistemas de obtención de imágenes con alta resolución permite la valoración no invasiva de la morfología ósea con evaluaciones tridimensionales más precisas, con las denominadas "biopsias virtuales" que permiten realizar la valoración del hueso en procesos de regeneración o remodelación. Este trabajo de revisión describe las características y limitaciones de la evaluación ósea de diferentes sistemas de imagen de alta resolución (Tomografia computerizada mediante radiación sincrotrón; Micro tomografía computerizada; Microscopio de escaneado acústico; Micro imagen por resonancia). También se describen diversas variables morfométricas que pueden ser obtenidas a partir de las imágenes obtenidas y que pueden ser comparadas con las variables histomorfométricas convencionales.
Palabras clave: Imagen alta resolución, biología ósea, biopsia virtual.
Introduction
Various studies have shown that bone resistance cannot be explained by the bone mass index alone (1). Determination of the bone mineral density (BMD) by dual-energy X-ray absorptiometry (DEXA) is inadequate to assess the biomechanical properties of bone. Other factors that appear to influence bone resistance are the trabecular microstructure, bone remodeling index, bone mass distribution, microfissure accumulation, mineral crystal quality, collagen fiber quality, and degree of mineralization (2).
Bone response to local mechanical stimuli was described in 1892 by Wolff (3), who established the hypothesis that "Every change in the form and function of bones, or of their function alone, is followed by certain definite changes in their internal architecture and equally definite secondary alteration in their external conformation, in accordance with mathematical laws". Although the relationship with possible mathematical laws has been questioned, it is not doubted that mechanical charges affect the internal organization of bone as well as its volume.
Recent advances in high-resolution imaging techniques, micro-magnetic resonance imaging (µMRI) and micro-computed tomography (µCT), permit evaluation of bone microarchitecture in in vitro samples (4). Assessment of morphometric values by means of two-dimensional histological images entails error accumulation due to structure overlap and the appearance of artifacts during sample preparation (jump cuts, excessive widths) (5). Development of new equipment for in vivo studies opens the way to so-called "virtual biopsies". These will offer more precise three-dimensional (3-D) bone morphometry studies compared with images obtained by invasive acquisition methods with bone biopsy fixation and embedding. Other image acquisition methods (scanning or transmission electron microscope, backscattered electron image, atomic force microscope, infrared spectroscopy, and Raman spectroscopy) have not been proposed for clinical application and were not considered in the present review.
Clinically, bone microarchitecture is assessed to predict bone fracture in patients with osteoporosis and to determine effects of pharmacological treatments (bisphosphonates) that cannot be evaluated by bone densitometry methods (4). In vitro images have been used to assess bone microstructure in maxillary bones (6), raising the prospect of using in vivo images to predict the prognosis of implantology patients and establish the optimal time for implant loading. We review the different high resolution imaging techniques used for bone biology assessment, describing the main morphometric parameters considered and their function in bone mechanobiology in general and in maxillary bones in particular.
High resolution imaging techniques
Three-dimensional assessment techniques must be adapted to the characteristics of bone microstructure. The thickness of spongy bone trabeculae is usually 100-150 µm separated by spaces of 500-1000 µm. If the resolution is not adjusted to trabecula size (sections thicker than 500 µm), variables obtained are considered "apparent" or as "texture analysis", since trabeculae can overlap and no direct access to the trabecular structure is possible (4, 6). Thus, conventional tomography methods, with a maximum resolution of 250 µm, cannot be used to study the average individual size of a trabecula (7).
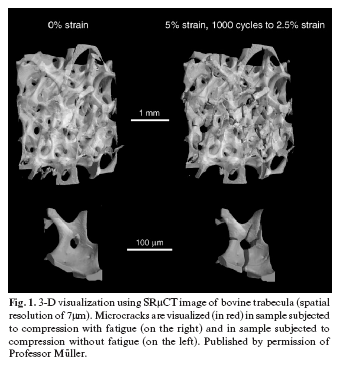
Synchrotron-Radiation Micro-Computed Tomography (SRµCT) is the reference method for assessing trabecular microarchitecture parameters and can produce resolutions of around 1 µm and even below the micrometric scale (nanoCT). Parallel monochromatic radiation (photons with the same energy that do not produce beam hardening) is used, with no geometric distortion and an optimal signal-noise ratio (8). It is based on the acceleration of electrons that generate a high amount of secondary radiation emission. More coherent, monochromatic and parallel light beams are obtained with this method than with conventional X-ray source, which improves the resolution and contrast of the final image. The system requires a high-cost infrastructure. Only equipment for use in in vitro studies is currently available. (Fig. 1) (9).
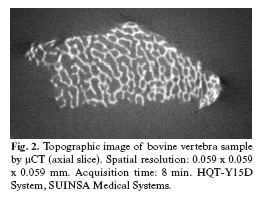
-Micro Computerized Tomography (µCT). Polychromatic radiation is generated (photons with different energy) and the beam diverges conically, which may produce geometric distortion, unlike in SRµCT, (8). The resolution obtained ranges from 7 to 20 µm depending on the equipment (Fig. 2). One difficulty concerns the appropriate setting of the differentiation threshold between mineral tissue and bone marrow, which can have a major effect on morphometric results. With polychromatic radiation, the center of the sample is more exposed to higher energy X-rays and has a darker appearance versus the periphery. Therefore, the gray level distribution is determined by sample size as well as by the degree of mineralization if a "beam hardening" correction is not included in the image reconstruction process (10).
With 14-µm resolution, the percentage difference between histomorphometry and µCT is 2.5% for bone volume/tissue volume (BV/TV) and 6.1% for trabecular separation (Tb.Sp) (11). It is at present an invasive method and can only be performed in small in vitro samples, although new equipment is being developed for use in small animals. This procedure has some shortcomings: a) The scintillation crystals that receive radiation contain defects that worsen with use, producing dark points that result in ring artifacts in the 3D reconstruction; b) an aliasing effect is produced by space resolution with a pixel size larger than the structure under study; and c) energy-dependent absorption produces beam hardening, generating refraction phenomena that can be partially corrected by reconstruction algorithms (5).
Using systems with lower resolution capacity is sometimes called micro-quantitative computed tomography (µQCT), which allows in vivo images to be obtained. It is used to assess bones in peripheral areas of limbs (distal radius, distal tibia or calcaneus) (pQCT). With current tomography equipment, spatial resolutions of about 250 µm are obtained, too high for the width of the trabecula (12).
Although resolutions of around 50 µ can be obtained with this technique, large X-ray doses (about 25± 360mSv) are required, which limits its use. Although a lower resolution is achieved compared with the above methods, µQCT can be used to obtain in vivo images of peripheral bone areas and for micro-finite element (µFE) studies (4).
- Scanning Acoustic Microscope (SAM). With this approach, the object to be visualized is explored with sound waves and the signal is digitally reconstructed in a single image. By this means, an image of the acoustic (or elastic) properties of a material is obtained, providing better definition of different types of tissue within the bone (mature and immature bone) as a function of their elastic properties. The main advantage of this method over direct biomechanical tests is its non-destructive character. Detected acoustic impedance (Z) can be assessed in 2-D with a resolution proportional to the wavelength used (25 µm for 50 MHz, and 2 µm for 900 MHz)(13). The main disadvantage versus the SRµCT technique is that a specific preparation of the sample is required and 3-D study is not possible. However, it offers quantitative assessment of the elastic properties of the material (13).
- Nanoindentation is a mechanical (not imaging) assessment technique that, like the above imaging technique, visualizes the elasticity properties of structures in the bone. It is based on conventional methods for estimating material hardness, making a mark on the material with a pyramidal diamond indenter (14).
- Micro-Magnetic Resonance Imaging (µMRI). The resonance signal has its origin in transitions between excited and relaxed states of protons in live tissue after being subjected to an electromagnetic field (6). In contrast to CT, images of bone tissue show high grayscale levels (near-black) close to background noise, whereas bone marrow shows low gray levels (near-white). One of the main drawbacks is that the gray level of any air bubble in the sample is similar to that of mineralized tissue (6). Image segmentation is especially difficult under these conditions, and the setting of an inappropriate threshold may lead to an under- or over-estimation of morphometric values.
Spatial resolutions of around 50 µm can be obtained but high magnetic fields are required. Development of this method is centered on the improvement of performance (obtaining magnetic fields of >10 tesla). To date, this technique has been limited to small in vitro samples of around 1 cm3, although, as with µCT, systems are being developed for in vivo studies of small animals (4).
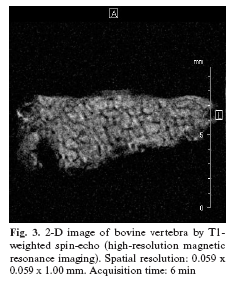
With this technique, resolutions of 80-200 µm and slice thicknesses of 300-700 µm are obtained (15). Acquisition of 3-D clinical images in vivo is impossible because of the time required to acquire the signal. However, this technique is useful for in vitro experiments and for the in vivo study of small experiment animals (6). (Fig. 3)
There are two main types of signal acquisition: gradient-echo and spin-echo sequences. The former has a higher probability of artifacts and therefore overestimation. Spin-echo has a better signal-noise ratio (SNR) but requires longer acquisition times, which can be compensated for by higher resonator power (16). The increase in magnetic field intensity (3 Tesla fields) in clinical equipment implies an increase in the signal-noise ratio, enhancing image quality, reducing acquisition time, and improving spatial resolution (16). In calcaneal cadaver bone, images obtained with a 3-T field showed a higher correlation with µ-CT values than did those obtained with a 1.5-T field (16).
Morphometric parameters with high resolution images
Histomorphometry and stereology are the conventional methods to assess bone microstructure. With these techniques, the sample is observed under light microscope with a resolution of around 1µm. In this case, 3-D measurements are made by inference, with the resulting errors derived from the anisotropic nature of trabecular bone. Reducing the number of errors in 3-D estimation requires serial sections and a higher sample volume, and a biopsy must be obtained (4). In contrast, conventional radiology can achieve a resolution of 40-µm thickness, and digital methods permit 2-D texture analyses but not 3-D evaluations.
Histomorphometric variables were originally designed for calculation from 2-D images acquired after the processing of bone tissue samples (17). Many variables used for conventional histomorphometry can be used with high resolution images, which offer greater precision for 3-D variables compared with conventional stereology techniques. Regardless of the 3-D reconstruction method used, the result is an image in grayscale. The information must be reduced in such a way that each voxel has a "bone" value or "marrow" value in the computer segmentation process (8).
- Bone Volume (Bone Volume/ Tissue Volume [BV/TV]): This 3-D parameter relates bone volume to total tissue volume. The value is obtained by adding together the volume of voxels within the triangles into which the 3-D surface is divided. The number of voxels with bone is then divided by the total number of voxels. When this measurement is taken from a 2-D image, the bone area is obtained (Bone Area/Tissue Area [B.Ar/T.Ar]) (6). This value is below 100% in trabecular bone but close to 100% in cortical bone (7). The BMD (Bone Mineral Density) can be obtained the voxels that contain bone by means of the different gray levels and mineralization (g/cm3), which represents the proportion of hydroxyapatite crystals (18).
- Bone Surface (Bone Surface/Bone Volume [BS/BV]). This 3-D parameter relates the bone surface to total bone volume and is obtained by triangulation of the trabecular surface. It increases with a decrease in the number of trabeculae. In patients with osteoporosis, the value of this ratio increases with progression of the disease (12, 19).
- Number of trabeculae (Trabecular Number [Tb.N],. This 2-D parameter can be calculated by using the Mean Intercept Length (MIL) method developed by Whitehouse (20). A grid of parallel lines is superimposed on the image. A ratio is obtained between the intersection points of the lines and the bone-marrow interface of the trabeculae, in relation to the total length of the grid-lines. This method can also be used to obtain trabecular separation (Tb.Sp) and trabecular thickness (Tb.Th) parameters, but it is not valid for 3-D assessment. These three variables can be obtained directly from a 3-D image by measurements in each voxel or with the system by the localization of spheres within and among trabeculae (21).
- Index of the structure of the model (Structure Model Index [SMI]). This variable indicates the prevalence of the rod-like or plate-like structures of the trabecular structure. It is obtained by differential analysis of the triangulated surface of a 3-D structure using a mathematical model. It is quantified from level 0 (plate-like structure) to level 3 (rod-like structure) (22). Osteoporosis is characterized by a shift from plate-like to rod-like structures of trabeculae.
- Degree of Anisotropy (DA). The concept of isotropy refers to the completely regular or irregular spatial orientation of a structure. Thus, bone that usually presents completely disoriented structures (trabeculae) combined with oriented structures (depending on the load) is considered anisotropic material (8). The DA can also be obtained from a 3-D image, as the ratio of the maximum to minimum radius of an ellipsoid created by the MIL method using a grid on all dimensions of the volume of interest. The DA increases with a decrease in the biomechanical resistance of bone and is reduced by the trabecular orientation caused by loads (isotropic orientation) (12, 23). It can also be obtained using the Volume Orientation (VO) method described by Odgaard (24).
- Bone Mineralization Ratio-Volume (BMR-V). This index of bone mineralization as a function of volume requires very high spatial resolution levels to distinguish between the different degrees of mineralization of bone and can be obtained by synchrotron radiation imaging. It is calculated by dividing the number of voxels with high mineralization by the number with low mineralization (19). This parameter has also been demonstrated to correlate with conventional histomorphometric bone turnover values (25).
- Number of Havers Canals/Cortical Bone Area (N.Ca/Ar). This variable is used to assess the porosity of cortical bone alone. A resolution of at least 20µm are required for its assessment, mainly by SAM or SRµCT (13).
Conclusions
- High-resolution image acquisition methods are not yet available in the clinical setting, and their use is limited to experiments with small animals or to the study of in vitro samples.
-The resolution obtained with magnetic resonance imaging does not achieve the results obtained with CT in bone. The development of higher power resonators could avoid CT radiation in in vivo studies of bone architecture.
References
1. Stenstrom M, Olander B, Lehto-Axtelius D, Madsen JE, Nordsletten L, Carlsson GA. Bone mineral density and bone structure parameters as predictors of bone strength: an analysis using computerized microtomography and gastrectomy-induced osteopenia in the rat. J Biomech. 2000 Mar;33(3):289-97. [ Links ]
2. McCreadie BR, Goldstein SA. Biomechanics of fracture: is bone mineral density sufficient to assess risk. J Bone Miner Res. 2000 Dec;15(12):2305-8. [ Links ]
3. Wolff J. The law of bone remodelling. Berlin: Springer; translation of the German 1892 edition.1986. [ Links ]
4. Lespessailles E, Chappard C, Bonnet N, Benhamou CL. Imaging techniques for evaluating bone microarchitecture. Joint Bone Spine. 2006 May;73(3):254-61. Epub 2006 Jan 13. [ Links ]
5. Bernhardt R, Scharnweber D, Muller B, Thurner P, Schliephake H, Wyss P, et al. Comparison of microfocus- and synchrotron X-ray tomography for the analysis of osteointegration around Ti6Al4V implants. Eur Cell Mater. 2004 Jun 30;7:42-51; discussion 51. [ Links ]
6. Choel L, Last D, Duboeuf F, Seurin MJ, Lissac M, Briguet A, et al. Trabecular alveolar bone microarchitecture in the human mandible using high resolution magnetic resonance imaging. Dentomaxillofac Radiol. 2004 May;33(3):177-82. [ Links ]
7. Moon HS, Won YY, Kim KD, Ruprecht A, Kim HJ, Kook HK, et al. The three-dimensional microstructure of the trabecular bone in the mandible. Surg Radiol Anat. 2004 Dec;26(6):466-73. [ Links ]
8. Odgaard A. Three-dimensional methods for quantification of cancellous bone architecture. Bone. 1997 Apr;20(4):315-28. [ Links ]
9. Peyrin F, Muller C, Carillon Y, Nuzzo S, Bonnassie A, Briguet A. Synchrotron radiation microCT: a reference tool for the characterization of bone samples. Adv Exp Med Biol. 2001;496:129-42. [ Links ]
10. Dalstra M, Verna C, Cacciafesta V, Andreassen TT, Melsen B. Micro-computed tomography to evaluate bone remodellingand mineralization. In: Majumbar S, Bay BK, editors. Noninvasive assesment of trabecular bone architecture and thecompetence of bone. New York: Kluwer academic-Plenum Publishers; 2001. p. 9-19. [ Links ]
11. Muller R, Van Campenhout H, Van Damme B, Van Der Perre G, Dequeker J, Hildebrand T, et al. Morphometric analysis of human bone biopsies: a quantitative structural comparison of histological sections and micro-computed tomography. Bone. 1998 Jul;23(1):59-66. [ Links ]
12. Muller R. Long-term prediction of three-dimensional bone architecture in simulations of pre-, peri- and post-menopausal microstructural bone remodeling. Osteoporos Int. 2005 Mar;16 Suppl 2:S25-35. Epub 2004 Aug 31. [ Links ]
13. Raum K, Leguerney I, Chandelier F, Talmant M, Saied A, Peyrin F, et al. Site-matched assessment of structural and tissue properties of cortical bone using scanning acoustic microscopy and synchrotron radiation muCT. Phys Med Biol. 2006 Feb 7;51(3):733-46. Epub 2006 Jan 19. [ Links ]
14. Zioupos P. In vivo fatigue microcracks in human bone: material properties of the surrounding bone matrix. Eur J Morphol. 2005 Feb-Apr;42(1-2):31-41. [ Links ]
15. Link TM, Majumdar S, Augat P, Lin JC, Newitt D, Lu Y, et al. In vivo high resolution MRI of the calcaneus: differences in trabecular structure in osteoporosis patients. J Bone Miner Res. 1998 Jul;13(7):1175-82. [ Links ]
16. Phan CM, Matsuura M, Bauer JS, Dunn TC, Newitt D, Lochmueller EM, et al. Trabecular bone structure of the calcaneus: comparison of MR imaging at 3.0 and 1.5 T with micro-CT as the standard of reference. Radiology. 2006 May;239(2):488-96. Epub 2006 Mar 28. [ Links ]
17. Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987 Dec;2(6):595-610. [ Links ]
18. Nuzzo S, Peyrin F, Cloetens P, Baruchel J, Boivin G. Quantification of the degree of mineralization of bone in three dimensions using synchrotron radiation microtomography. Med Phys. 2002 Nov;29(11):2672-81. [ Links ]
19. Borah B, Dufresne TE, Ritman EL, Jorgensen SM, Liu S, Chmielewski PA, et al. Long-term risedronate treatment normalizes mineralization and continues to preserve trabecular architecture: sequential triple biopsy studies with micro-computed tomography. Bone. 2006 Aug;39(2):345-52. Epub 2006 Mar 29. [ Links ]
20. Whitehouse WJ. The quantitative morphology of anisotropic trabecular bone. J Microsc. 1974 Jul;101(Pt 2):153-68. [ Links ]
21. Fajardo RJ, Muller R. Three-dimensional analysis of nonhuman primate trabecular architecture using micro-computed tomography. Am J Phys Anthropol. 2001 Aug;115(4):327-36. [ Links ]
22. Hildebrand T, Ruegsegger P. Quantification of Bone Microarchitecture with the Structure Model Index. Comput Methods Biomech Biomed Engin. 1997;1(1):15-23. [ Links ]
23. Goulet RW, Goldstein SA, Ciarelli MJ, Kuhn JL, Brown MB, Feldkamp LA. The relationship between the structural and orthogonal compressive properties of trabecular bone. J Biomech. 1994 Apr;27(4):375-89. [ Links ]
24. Odgaard A, Jensen EB, Gundersen HJ. Estimation of structural anisotropy based on volume orientation. A new concept. J Microsc. 1990 Feb;157(Pt 2):149-62. [ Links ]
25. Borah B, Ritman EL, Dufresne TE, Jorgensen SM, Liu S, Sacha J, et al. The effect of risedronate on bone mineralization as measured by micro-computed tomography with synchrotron radiation: correlation to histomorphometric indices of turnover. Bone. 2005 Jul;37(1):1-9. [ Links ]
![]() Correspondence:
Correspondence:
Dr. Jorge Cano Sánchez
Departamento de Medicina y Cirugía Bucofacial
Facultad de Odontología (UCM).
Avda Complutense s/n 28040 Madrid, Spain
Email: jo.cano@wanadoo.es
Received: 26-07-2006
Accepted: 10-06-2007