INTRODUCTION
Teicoplanin is an antibacterial glycopeptide against gram+ cocci whose clinical efficiency correlates with permanence over the MIC, especially with the AUC24h/MIC value. For this reason, it is important to monitor trough concentration and individually adjust doses to keep trough levels above 10 µg/mL. Teicoplanin offers the ideal pharmacokinetic characteristics (t1/2: 100-170h) to be used by hospital home care units (HHCU) in an ambulatory setting.
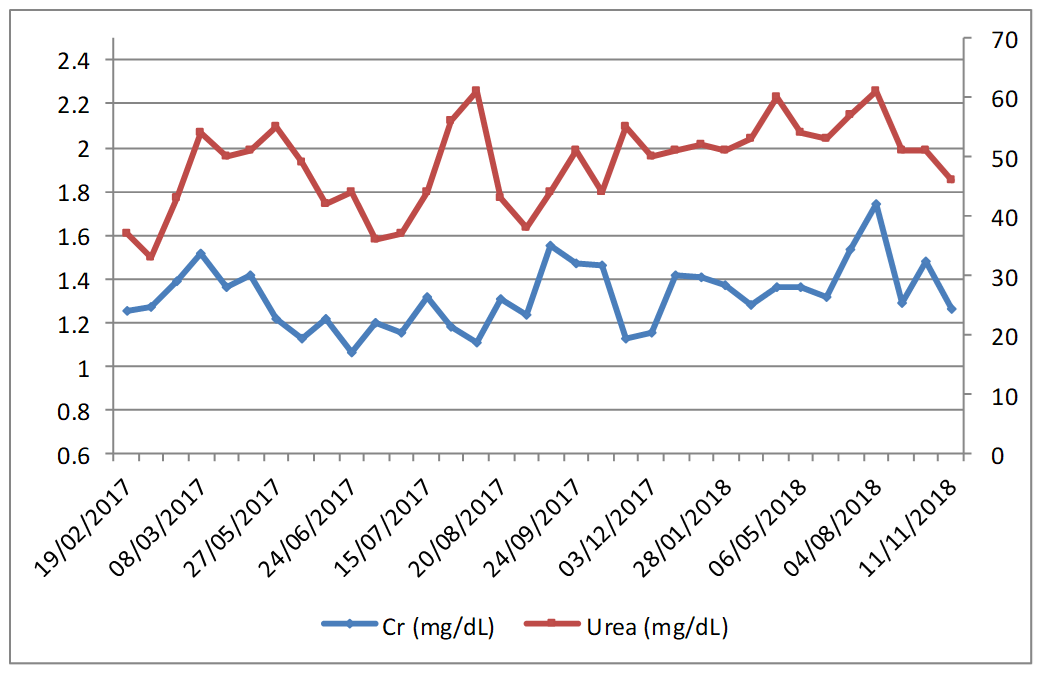
We present a case of an 83-year-old man with high blood pressure, dyslipidaemia, COPD, hypertensive cardiopathy, brady-tachycardia syndrome with paroxysmal atrial fibrillation, and heart failure and secondary respiratory episodes, chronic kidney failure and hypothyroidism.
In June 2014, he came to our hospital about his left knee infection, operated on 10 occasions. It was verified that he had arthrodesia infection, and that two fistulae were present for which treatment with cotrimoxazole was administered. Amputation was considered, but the patient preferred chronic antibiotic treatment and fistula cleansing. He remained stable until July 2016, when the fistulae became worse and withdrawing the osteosynthesis material by surgical cleaning was considered. He underwent surgery in February 2017 and a stud from the arthrodesia was removed, while a spacer with vancomycin and gentamicin was placed. While still in hospital, he started treatment with rifampicin and vancomycin, plasma concentration was monitored and the individual dose was adjusted.
A microbiology examination revealed the presence of hetero-resistant Staphylococcus epidermidis strains, which meant the possibility of being treated only with linezolid or glycopeptides. Traumatologist indicated chronic suppressive antibiotic treatment given the risk of infection persisting, and no further surgery was indicated due to the patient's high comorbidity. The patient was placed on oral linezolid treatment, 600 mg/12h, but because of his chronic treatment indication, other options were assessed by the multidisciplinary team involved (geriatrician, traumatologist, a doctor from HHCU and a pharmacist) since haematological1 and neuropathic2 toxicity, along with high cost3, (although now, with the generic presentation this is not a problem) would limit its chronic use. After being on linezolid treatment for two and a half months, treatment was changed to teicoplanin, administered through the HHCU at the patient's home, along with pharmacokinetic monitoring in the Pharmacy Service. The guidelines of the BSAC European OPAT Summit Conference of March 2011, by the Greater Glasgow and Clyde NHS, were followed. This consisted in administering a loading dose according to creatinine clearance and the patient's weight, followed by a dispensing plan of 3 days/week (Mondays, Wednesdays and Fridays) with dose based on kidney function. After the initial phase, trough levels were first determined, which fell within the therapeutic range (trough level: 15.5 µg/mL). This pattern was later altered to two days/week (Mondays and Thursdays) and the trough concentration (trough level) after 3 weeks was 10.7 µg/mL. At this point of the treatment, a decision was made to ensure viable treatment and one that the patient would accept, which involved attempting weekly administering teicoplanin. A search in the literature was done and one similar clinical case4 was found. A decision was made to weekly administer 2,800 mg of teicoplanin, and to monitor trough level fortnightly and kidney function weekly, plus doing a blood count and monitoring inflammation parameters (CRP/ESR). During the 18-month follow-up, the mean trough level value for teicoplanin was 16.03 µg/mL (within the range of 9.2-24.6 µg/mL; figure 1). At the beginning, doses were adjusted according to trough level and kidney function to target 15 µg/mL. Then we incorporated the population pharmacokinetic model published by Ogawa et al.5 and through the NONMEM programme, v.7.3.0 (non-linear mixed effects model). Individual PK parameters were estimated with the Bayesian approach, with which different dose adjustment scenarios were simulated to help with decision making. The patient's evolution has been satisfactory as no sign of inflammation has appeared and the fistulae cured. Two hospitalisations for decompensation of the patient's heart pathology with high associated CRP/ESR (Figure 1) are noteworthy, but his knee was inflammation-free. Figure 2 illustrates kidney function evolution in creatinine and serum urea terms. His worsened condition in summer was due to dehydration from not drinking enough water. This was corrected, baseline levels were restored, and no significant worsening was evidenced. Haematologically speaking, the values for the following remained stable; haemoglobin (mean:12.7 g/dL range: 11.8-13.6 g/dL), leukocytes (mean: 5.7x103 range: 4.4-7.2x103) and platelets (mean:180x103 range:122-271x103); and no toxicity was seen. The patient is still (November 2018) on the weekly intravenous teicoplanin treatment, adjusted according to individual PK parameters, estimated to obtain a trough level of 10-15 µg/mL. Based on this clinical case and the aforementioned one, 4 teicoplanin, along with pharmacokinetic and toxicity monitoring, is an alternative in patients with chronic osteoarticular infection by Gram+ in whom treatment with oral antibiotics for toxicity and/or resistances is not feasible.
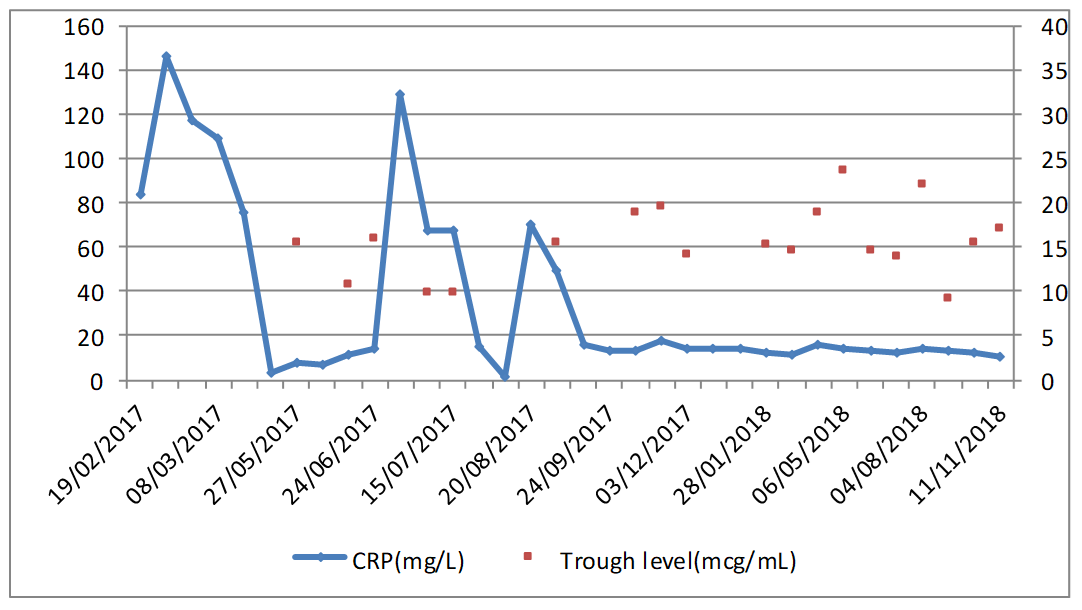
Figure 1. Temporal evolution of the inflammation parameters and of the trough concentration for teicoplanin.