My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Pharmacy Practice (Granada)
On-line version ISSN 1886-3655Print version ISSN 1885-642X
Pharmacy Pract (Granada) vol.6 n.3 Redondela Jul./Sep. 2008
| Original Research |
Pilot assessment of patient satisfaction and clinical impact of Medicare Part D in diabetic geriatric patients
Sandra L. KIM, Daniel R. TOUCHETTE, Jo A. STUBBINGS, Anne M. SCHULLO-FEULNER, Karen S. PATER.
| ABSTRACT Objectives: To assess patients 1) satisfaction with their decision to enroll or not enroll in the Medicare Part D program, and 2) clinical status of diabetes before and after decision to enroll in Medicare Part D. Key words: Medicare Part D. Aged. Diabetes Mellitus. United States. | RESUMEN Objetivos: Evaluar 1) la satisfacción de los pacientes con la decisión de incluirlos o no en el programa de Medicare Part D, y 2) el estado clínico de la diabetes antes y después de incluirlos en el Medicare Part D. Palabras clave: Medicare Part D. Ancianos. Diabetes Mellitus. Estados Unidos. |
Sandra L. KIM. PharmD. Clinical Pharmacist/Assistant Professor. College of Pharmacy, University of Illinois at Chicago. Chicago, IL (USA).
Daniel R. TOUCHETTE. PharmD, M.A. Assistant Professor. College of Pharmacy, University of Illinois at Chicago. Chicago, IL (USA).
Jo Ann STUBBINGS. RPh, MHCA. Manager, Research and Public Policy, Ambulatory Care Pharmacy Department. Clinical Assistant Professor. Department of Pharmacy Practice. Center for Pharmacoeconomic Research. College of Pharmacy, University of Illinois at Chicago. Chicago, IL (USA).
Anne Marie SCHULLO-FEULNER. PharmD, BCPS. Clinical Education Coordinator and Assistant Professor. College of Pharmacy, University of Minnesota. Heart & Vascular Clinical Specialist, Park Nicollet Methodist Hospital. Minneapolis, MN (USA).
Karen S. PATER. PharmD, BCPS, CDE. Assistant Professor. School of Pharmacy, University of Pittsburgh, Pittsburgh, PA (USA).
Received (first version): 2-Jun-2008
Accepted: 18-Jul-2008
INTRODUCTION
According to the Centers for Disease Control (CDC), the total number of individuals aged 65 years and older with a diagnosis of diabetes more than doubled from 2.3 million to 5.8 million from 1980 to 2004.1 As the number of people diagnosed with diabetes increase each year and as newer, more expensive medications are introduced into the market, the cost of multidrug anti-diabetes therapy is becoming burdensome. Therefore, there is a growing need for improved prescription drug coverage. Expanding medical coverage to help meet this need for eligible persons has been a State and Federal cooperative venture. In 2003 the Medicare Prescription Drug Improvement and Modernization Act (MMA) was passed, allowing for the coverage of outpatient prescription drugs, insulin, and medical supplies associated with injections.2 The Centers for Medicare and Medicaid Services (CMS) has issued guidelines for prescription drug plan formularies to include at least two drugs in each approved category and class (oral anti-diabetes agents and insulin).3
Background
The economic burden of healthcare impacts the individuals access to medications and their chronic condition.4-9 Studies have shown that patients with limited access to prescription drugs due to reduced insurance coverage demonstrate significantly decreased compliance and increased morbidity.10-12 One intent of Medicare Part D is to increase access to outpatient prescription drugs for the elderly by reducing cost-related barriers. Experience with the application of Medicare Part D varies among patients and clinical settings. The purpose of this study is to assess patient satisfaction with their Medicare Part D enrollment decision as well as evaluate clinical impact in geriatric patients with diabetes who enrolled in Medicare Part D.
Primary outcomes of this study were patient satisfaction with Medicare Part D enrollment decision and glycosylated hemoglobin (HbA1c), low-density lipoprotein (LDL), and blood pressure in patients with or without Medicare Part D.
METHODS
Study Design
This study consisted of a survey of patients with and without Medicare Part D and a retrospective chart review of geriatric patients with diabetes with or without Medicare Part D. The protocol was approved by the Institutional Review Board and all patients provided informed consent. Patients were included in the study if they were 65 years or older and either enrolled or did not enroll in Medicare Part D by May 1, 2006. Non-English speaking patients were included if a translator was present at the time of the survey. Patients were excluded if they had diabetes with documented active cancer, were undergoing hemodialysis, or were unable to answer survey questions due to impaired cognition. Pharmacists in the clinical setting obtained written consent before administering the survey to patients. All patients, regardless of diabetes status, were asked questions regarding their satisfaction with their decision to enroll or not enroll into Medicare Part D. Responses to the survey were anonymous unless the patient was identified as having diabetes. A retrospective chart review was then conducted only in patients with diabetes to obtain HbA1c, LDL, and systolic and diastolic blood pressures before and after the Medicare Part D enrollment.
Data Collection
Surveys assessing satisfaction with prescription drug insurance enrollment decisions were administered to patients by clinical pharmacists in a variety of clinic settings November 2006 through December 2006. All patients, regardless of diabetes status, were surveyed regarding their satisfaction with their decision to enroll or not to enroll into Medicare Part D. Data collected from the survey included gender, race, age, current prescription coverage, enrollment status (enrolled or not), enrollment date (if known), prior prescription coverage before enrolling into Medicare Part D, reason for enrolling or not enrolling into Medicare Part D, general satisfaction with their decision for enrolling or not enrolling into Medicare Part D, and diagnosis of diabetes.
For subjects identified from the survey as having diabetes, a retrospective chart review was conducted via electronic medical record. Data from chart review was compiled and analyzed comparing HbA1c, LDL concentrations and blood pressure. Pending subjects enrollment date, data was collected 6 months prior to enrollment (pre-enrollment phase) and 6 months after enrollment (post-enrollment phase). Dual eligible patients qualified for both Medicaid and Medicare and were automatically enrolled into Medicare Part D on January 1, 2006.13 All other patients were required to enroll by Medicare Part D by May 1, 2006. Pre-enrollment values were obtained in the 6 months prior to the start of Medicare Part D enrollment (July 1 - December 31, 2005). Post-enrollment values were obtained after enrollment was complete for the 2006 year (May 1 - October 31, 2006). Only the last available value was used for the analysis. Goal values for HbA1c, LDL, and blood pressure were defined using the American Diabetes Associations recommended standards (HbA1c less than 7%; LDL less than 70 mg/dL; systolic blood pressure less than 130; and diastolic blood pressure less than 80).14 HbA1c and LDL values were not available for all patients due to noncompliance with clinician visits.
Statistical Analysis
The sample size was calculated from the primary study outcomes of change in HbA1c, LDL, and blood pressure. Setting alpha=0.05 and beta=0.2 and assuming standard deviations of 1.2% and 10 mmHg respectively, the required sample size was calculated to be 33 for detecting differences of 0.6% for HbA1c and 5 mmHg for diastolic and systolic blood pressure. Assuming a standard deviation of 15 mg/dL, the required sample size was 20 for detecting changes in LDL of 20 mg/dL.
Descriptive statistics were used to summarize findings from the satisfaction survey. Paired t-tests were used to determine the effects of Medicare Part D on clinical outcomes from the pre-implementation to the post-implementation periods. A p-value of 0.05 was considered statistically significant.
RESULTS
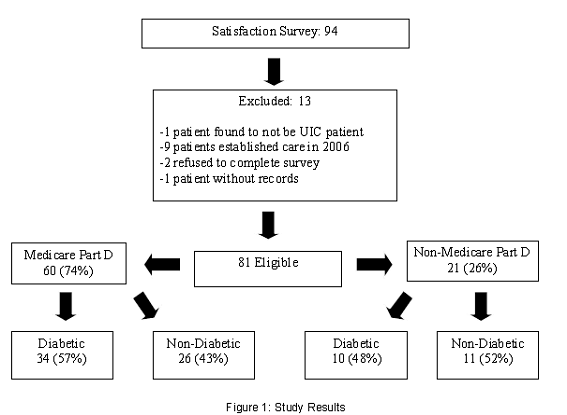
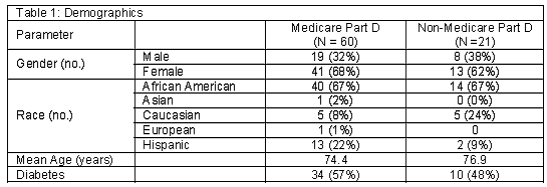
Of the 94 patients surveyed for this study, 81 were eligible for enrollment. Figure 1 shows a detailed breakdown of patient participation in the study and reasons for exclusion. Thirteen patients were excluded for a variety of reasons: one patient was found not to be a patient at the facility, two patients refused to complete the survey, one patient did not have records at the facility, and nine patients had established care at the facility after the pre-enrollment phase. The demographic information for participating patients is shown in Table 1.
Satisfaction Survey
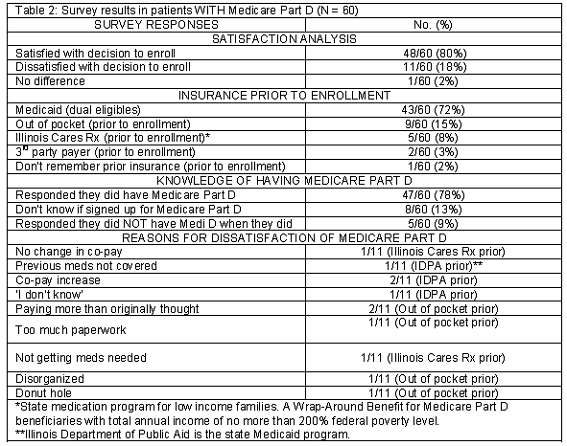
Survey results showed that 60 patients were enrolled into Medicare Part D, and 21 patients were not enrolled. Table 2 summarizes patient satisfaction with their enrollment decision regarding Medicare Part D. Of the 60 patients who were enrolled into Medicare Part D, 48 patients responded that they were satisfied with their decision. If they were unsure that they had Medicare Part D, they were asked if they were satisfied with their current prescription insurance. Of the 60 patients who were enrolled into Medicare Part D, 43 had Medicaid prior to enrollment. Their co-pays, however, did change slightly from paying 0USD to 1USD for generic medications, but remained at 3USD for brand name medications. Seven patients with Medicare Part D had a third party payer prior to enrollment and 9 patients paid out of pocket prior to enrollment.
Of the 9 patients who paid out of pocket prior to enrollment, 3 of them were satisfied with having enrolled into Medicare Part D. Five patients were dissatisfied due to the following reasons: no change in co-pay, discontinuation of medication coverage, increase in co-pay, a higher co-pay than the expected amount, the amount of paperwork involved, and program disorganization. Interestingly, the people who responded that their co-pay increased or that their medication was no longer covered were dual eligible patients. Increased co-pays and specific medication coverage issues were not assessed due to limited resources. In addition, one person had already reached the donut hole and was not satisfied with having Medicare Part D. The donut hole is the period of no coverage that in 2006 started when total drug expenditure equaled 2400USD and ended when total out-of-pocket costs equaled 3850USD15.
Not only was patient satisfaction with Medicare Part D assessed, but also the patients knowledge of their enrollment into Medicare Part D. Eight patients who had Medicare Part D were unaware of their enrollment status, 5 patients stated they did not have Medicare Part D when they actually did, and 47 patients correctly stated that they have Medicare Part D.
There were a variety of reasons for patients choosing not to sign up for Medicare Part D, as shown in Table 3. Out of the 21 patients who did not sign up for Medicare Part D, 7 did not know what it was and 7 already had a prescription drug plan they were satisfied with. Three patients responded that Medicare Part D was confusing. Two people did not think they would save money with Medicare Part D and one person thought there was too much paperwork involved. There was one person who did not sign up because no one helped him.
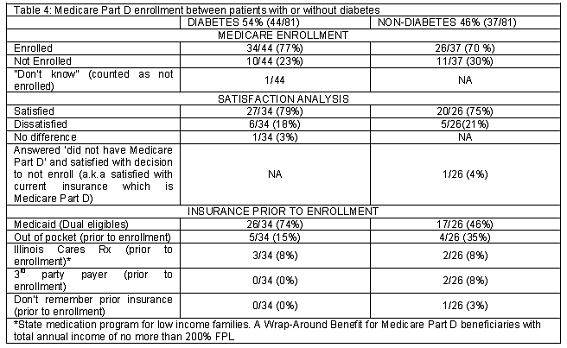
The data was also analyzed to compare enrollment rate and satisfaction with the enrollment decision regarding Medicare Part D amongst patients with and without diabetes (Table 4). A slight trend was observed in that patients with diabetes were more likely to be enrolled into Medicare Part D and were more satisfied with their Medicare Part D enrollment decision than patients without diabetes.
Clinical Outcomes
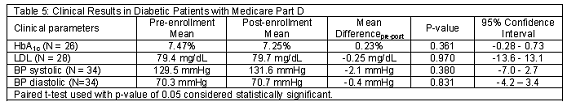
Clinical outcomes were unchanged in the pre-enrollment to the post-enrollment periods as seen in Table 5. Twenty six patients had evaluable HbA1c levels in both time periods. Mean HbA1c was 7.47% in the pre-enrollment period and 7.25% post-enrollment (differencepre-post = 0.23; 95% CI = -0.28 to 0.73%). Twenty eight patients had LDL assessments done in each of the two periods. There was no change in LDL in the two time periods (pre = 79.4 mg/dL; post = 79.7; differencepre-post = -0.25; 95%CI = -13.6 to 13.1). Similarly, of the 34 patients with blood pressure measurements in both time periods, there were no significant differences observed in the two time periods. Mean systolic blood pressure was 129.5 in the pre-enrollment period and 131.6 in the post-enrollment period (differencepre-post = -2.1; 95%CI = -7.0 to 2.7). Mean diastolic blood pressure was 70.3 for the pre-enrollment period and 70.7 for the post-enrollment period (differencepre-post = -0.4; 95%CI = -4.2 to 3.4).
DISCUSSION
Survey results showed that 80% of the people who were enrolled into Medicare Part D were satisfied with their decision to enroll. This satisfaction rate was also found in a nationwide telephone survey sponsored by AARP in October 2007 and conducted in 400 patients 65 years or older. Eighty five percent of the individuals stated they were satisfied with their Medicare Part D prescription drug plan. However, it is unknown what percent of these surveyors were dual eligible patients.16 The high satisfaction rate in this study may be due to the fact that majority of Part D patients were dual eligible patients and experienced little change in their co-payments or coverage with Medicare Part D. There were three dual eligible patients, however, who stated they did experience a change in co-pay or formulary. The co-payment for generic drugs for dual eligible patients increased from 0USD under Medicaid to 1USD under Medicare Part D. They experienced a change in formulary because medications previously covered under the Illinois State Medicaid program were no longer covered under Medicare Part D. Examples of this change in coverage would be certain over the counter medications and benzodiazepines, which are not Part D drugs. For dual eligible patients, any drug not covered by Medicare Part D may be covered by Medicaid if it is covered for the non-dual eligible population in the state. It would have been ideal to review each patients medication list to determine the medications that were changed; however, due to the fact that many of the patients used an external pharmacy, or more than one pharmacy, it was not feasible to determine an accurate medication list.
Several issues with Medicare Part D were identified through responses from patients who reported that they were dissatisfied with their decision to enroll and from patients who did not sign up for Medicare Part D. One common theme between both groups of patients was that there was too much paperwork and too many requirements involved to enroll into Medicare Part D. Patients did not understand how to fill out the paperwork and could not track down the appropriate paperwork necessary to show proof of the requirements that were necessary to meet. Another issue with the enrollment process is that patients did not know what the Medicare Part D prescription plan was and therefore, did not sign up. Many of the patients who did not enroll stated that they have never heard of it, or that they did hear of it but did not understand it. This raises an issue regarding enrollment process and how it needs to be further simplified for patients. A national survey also found people who were not auto enrolled stating it was a confusing process. Thirty three percent of the enrolled patients (N=776) agreed that the enrollment process was very complicated and 52% agreed they had difficulty understanding how Medicare Part D works and what savings it would provide.17
In addition to the issues of confusion with the paperwork and complications with the enrollment process, there was also confusion as to patient awareness of their enrollment into Medicare Part D, namely amongst the dual eligible patients. Twenty two percent of the patients with Medicare Part D responded that they either did not know they had Medicare Part D or that they did not have Medicare Part D, despite the fact that they were enrolled. Patient enrollment was confirmed when the patient showed their prescription drug card or when their registration form showed that they had Medicaid and Medicare, which indicates that they are dual eligible patients. Because these patients were automatically enrolled, they did not know that they were enrolled into Medicare Part D. Their lack of awareness suggests there is also confusion with the auto enrollment process.
The coverage gap, also known as the donut hole, was another issue raised by one patient who stated they were dissatisfied with their decision to enroll in Medicare Part D. This patient was paying out of pocket when he enrolled into Medicare Part D and by December 2006, he had entered the donut hole. A recent national survey in individuals 65 years and older with diabetes showed that a large proportion of older adults may exceed the initial coverage limit under the Medicare Part D drug benefit. The survey found that the mean annual drug expenditure for geriatric patients with diabetes was 4,171USD and that more than 60% of the individuals eligible to enroll into Medicare Part D are predicted to exceed the initial coverage limit and fall into the donut hole. During the time of our surveys, the coverage limit was 2,250USD.18 As many more patients enter the donut hole, they may be more likely to reduce or discontinue their medications, which will lead to worsening control of their chronic illnesses.19,20 A recent study investigated the effect of pharmacy benefit caps on chronic illnesses and found that benefit caps were associated with higher rates of medication discontinuation. It can be inferred from this study that patients who reach the donut hole in Medicare Part D may be more likely to discontinue their medication. It was also found that use of antihypertensives, antihyperlipidemics and antidiabetics was 15-28% lower among patients who were in a capped plan versus noncapped lans.11
The concerns identified regarding patients enrolling in an appropriate plan and educating them about the plan raises a need for improvement in the enrollment process of Medicare Part D. One way to alleviate the confusion is to increase education amongst health care professionals. Patients interact with a variety of health care professionals and with their increased knowledge of Medicare Part D, they are able to communicate with and assist patients when questions arise. Another suggestion is that during the enrollment period health care professionals be trained to ask patients who are 65 years and older if they are enrolled into Medicare Part D yet. Health care professionals may not feel comfortable asking their patients about Medicare Part D if they dont understand it themselves. A national survey conducted by the Kaiser Family Foundation found that 91% of the pharmacists and 92% of the physicians agreed that the Medicare prescription drug benefit is too complicated.21 Improved communication and education to health care professionals is necessary to increase awareness of Medicare Part D to patients.
We had initially anticipated that the prescription drug coverage added by the Medicare Part D insurance would lead to an improvement in clinical parameters such as HbA1c, LDL and BP in patients with diabetes. However, due to the large proportion of dual eligible patients (72% in our analysis), prescription coverage did not change for many. For many others, higher copayments, entry into the donut hole, and changes to covered medications may have adversely impacted their ability to afford their medications or complicated their therapy. As such, we did not observe a statistically significant difference in any of the clinical parameters evaluated. This implies that this policy does not affect patients clinical condition or that the policy had a mixed effect, benefiting some patients and being detrimental to others, with no discernable net change.
Limitations
There are many limitations to this study. One limitation was the studys small sample size. We were unable to recruit sufficient numbers of patients with HbA1c and LDL values in both time periods of the study. Frequent measurement of LDL and HgA1c in patients with diabetes is a well-documented problem.22,23
The pre-post design is both a strength and a limitation of this study. This design allowed us to enroll a fewer number of patients yet still have sufficient power to detect clinically meaningful changes in clinical parameters. However, a time bias was introduced by not having a concurrent control group. Since a randomized crossover study was not possible, different events occurring during the two time periods could not be controlled or their potential bias eliminated. Although we cannot confirm that any such events (such as a diabetes education program) occurred in some patients during the study period, we cannot deny that they occurred either.
It would have been ideal to obtain medication lists and review changes in medications after enrollment into Medicare Part D; however, it was not feasible to determine changes in medications since patients filled their prescriptions outside of the facility. Medication changes occurring as a result of patient enrollment in a medication insurance plan may have been responsible for some of the observed effects. We were unable to determine to what degree these changes may have impacted therapy, satisfaction, and outcomes.
Several different pharmacists administered the satisfaction survey, which may have introduced surveyor bias. Patients may have felt prompted to answer the questions in a certain way depending on how it was asked, or, the surveyor may have recorded what insurance the patient actually had rather than what the patient responded.
The short term nature of the study is also a limitation since it may not have been enough time to see much improvement in patients clinical status and also, because it does not capture the realistic, long term effects of Medicare Part D (e.g. donut hole). By December 2006, one person already fell into the donut hole but if the survey was extended longer and included patients in Medicare Part D for the entire year, more patients in the donut hole may have been interviewed.
The fact that majority of the population at this facility are Medicaid patients also introduces biases into the study. Because the majority of the patients are dual eligible, we are not able to see the true effect of Medicare Part D on patients who previously paid out of pocket. The true advantage of Medicare Part D would be expected in a previously cash paying population. However, because many of the patients are dual eligible, the high satisfaction rate is what would be expected in this patient population due to the fact that their co-pays and formularies are not expected to change.
CONCLUSIONS
The majority of patients in this study were dual eligible patients and were satisfied with their decision to enroll in Medicare Part D, which coincides with the fact that they did not experience a significant change in co-payment or formulary. The confusion with the Medicare Part D enrollment process indicates that there needs to be improvement in communication with patients about Medicare Part D and greater education amongst health care professionals. Due to the small sample size and lack of power of the study, it cannot be concluded that Medicare Part D has greater improvement in HbA1c, LDL and BP compared to patients without Medicare Part D. However, there was a trend of improvement in these clinical parameters in patients with Medicare Part D and who were uncontrolled in their diabetes. More longitudinal studies are necessary to capture the true effect of Medicare Part D on the impact of diabetes.
ACKNOWLEDGEMENTS
Satisfaction survey: Larisa H. Cavallari, PharmD; Shiyun Kim, PharmD, CDE; Mary Ann Kliethermes, B.S., PharmD; Jessica Michaud, PharmD, BCPS; Edith Nutescu, PharmD, FCCP; Annette N. Pellegrino, PharmD, BCPS; Nancy L. Shapiro, PharmD, BCPS; Daphne E. Smith, PharmD, CDE; Jessica Tilton, PharmD. Statistical advice: Mathew Thambi, PharmD, MPH, BCPS; Sonia Ibrahim, PharmD, BCPS.
CONFLICT OF INTEREST
There was no external funding for this research, and the authors report no conflicts of interest related to the subject of this manuscript.
| References |
1. Centers for Disease Control, National Diabetes Surveillance System. Available at: http://www.cdc.gov/diabetes/statistics/prev/national/tnumage.htm. Accessed August 17, 2006. [ Links ]
2. Ashkenazy R, Abrahamson, M. Medicare Coverage for Patients with Diabetes. J Gen Intern Med. 2006;21:386-392. [ Links ]
3. Centers for Medicare and Medicaid. Formulary Review Guidelines. Available at: http://www.cms.hhs.gov/PrescriptionDrugCovContra/03_RxContracting_FormularyGuidance.asp#TopOfPage. Accessed August 17, 2006. [ Links ]
4. Piette J, Wagner T, Potter M, Schillinger D. Health insurance status, cost-related medication underuse, and outcomes among diabetes patients in three systems of care. Med Care. 2004;42(2):102-109. [ Links ]
5. Soumerai SB, Pierre-Jacques M, Zhang F, Ross-Degnan D, Adams AS, Gurwitz J, Adler G, Safran DG. Cost-related medication nonadherence among elderly and disabled Medicare beneficiaries. Arch Intern Med. 2006;166(17):1829-1835. [ Links ]
6. Piette J, Heisler M, Wagner T. Cost-related medication underuse among chronically ill adults: the treatments people forgo, how often, and who is at risk. Am J Public Health. 2004;94(10):1782-1787. [ Links ]
7. Spertus J, Decker C, Woodman C, House J, Jones P, OKeefe J, Borkon AM. Effect of difficulty affording health care on health status after coronary revascularization Circulation. 2005;111(20):2572-2578. [ Links ]
8. Kennedy J, Coyne J, Sclar D. Drug affordability and prescription noncompliance in the United States: 1997-2002. Clin Ther. 2004;26(4):607-614. [ Links ]
9. Shinogle J, Wiener J. Medication use among Medicaid users of home and community-based services. Health Care Financ Rev. 2006;28(1):103-106. [ Links ]
10. Jackson J, Doescher M, Saver B, Fishman P. Prescription drug coverage, health, and mediation acquisition among seniors with one or more chronic conditions. Med Care. 2004;42(11):1056-1065. [ Links ]
11. Joyce G, Goldman D, Karaca-Mandic P, Zheng Y. Pharmacy benefit caps and the chronically ill. Health Aff. 2007;26(5):1333-1344. [ Links ]
12. Steinman M, Sands L, Covinsky K. Self-restriction of medications due to cost in seniors without prescription coverage. J Gen Intern Med.2001;16(12):793-799. [ Links ]
13. Centers for Medicare and Medicaid. Auto-Enrollment and Facilitated Enrollment of Low Income Populations. Available at: www.cms.hhs.gov/States/Downloads/AutoversusFacilitatedEnrollment.pdf. Accessed October 29, 2007. [ Links ]
14. American Diabetes Association. Standards of Medical Care in Diabetes. Diabetes Care. 2006; 29:S4-S42. [ Links ]
15. Centers for Medicare and Medicaid. Bridging the Coverage Gap. Available at: ttp://www.cms.hhs.gov/PrescriptionDrugCovGenIn/01a_bridgingthegap.asp#TopOfPage. Accessed November 21, 2007. [ Links ]
16. Keenan, Teresa. Prescription drugs research report. 2007 November. Available at: ttp://www.aarp.org/research/health/drugs/rx_medicare d.html. Accessed April 20, 2008. [ Links ]
17. Heiss F, McFadden D, Winter J. Who failed to enroll in Medicare Part D, and why? Early results. Health Aff (Milwood). 2006;25(5):w344-354. [ Links ]
18. Tjia J, Schwartz JS. Will the Medicare prescription drug benefit eliminate cost barriers for older adults with diabetes mellitus? J Am Geriatr Soc. 2006;54(4):606-612. [ Links ]
19. Hsu J, Fung V, Price M, Huang J, Brand R, Hui R, Fireman B, Newhouse JP. Medicare beneficiaries' knowledge of Part D prescription drug program benefits and responses to drug costs. JAMA. 2008;299(16):1929-1936. [ Links ]
20. Madden JM, Graves AJ, Zhang F, Adams AS, Briesacher BA, Ross-Degnan D, Gurwitz JH, Pierre-Jacques M, Safran DG, Adler GS, Soumerai SB. Cost-related medication nonadherence and spending on basic needs following implementation of Medicare Part D. JAMA. 2008;299(16):1922-1928. [ Links ]
21. Kaiser Family Foundation/Harvard School of Public Health survey. Seniors and the Medicare Prescription Drug Benefit. Available at: www.kff.org. Accessed April 26, 2007. [ Links ]
22. Fuke D, Hunt J, Siemienczuk J, Estoup M, Carroll M, Payne N, Touchette D. Cholesterol management of patients with diabetes in a primary care practice-based research network. Am J Manag Care. 2004;10(2 Pt 2):130-136. [ Links ]
23. Mangione CM, Gerzoff RB, Williamson DF, Steers WN, Kerr EA, Brown AF, Waitzfelder BE, Marrero DG, Dudley RA, Kim C, Herman W, Thompson TJ, Safford MM, Selby JV; TRIAD Study Group. The association between quality of care and the intensity of diabetes disease management programs. Ann Intern Med. 2006:145(2):107-116. [ Links ]