Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Pharmacy Practice (Granada)
versión On-line ISSN 1886-3655versión impresa ISSN 1885-642X
Pharmacy Pract (Granada) vol.6 no.3 Redondela jul./sep. 2008
| Original Research |
Patient-reported outcomes of therapy with two brands of ibuprofen
Lucky L. NWIDU, Joshua F. ENIOJUKAN, Azuka C. OPARAH.
| ABSTRACT Objective: To investigate patients reported outcome following medication with two brands of 400 mg ibuprofen used to alleviate musculoskeletal pains. Key words: Therapeutic Equivalency. Treatment Outcome. Ibuprofen. Nigeria. | RESUMEN Objetivo: Investigar los resultados comunicados por los pacientes después de la medicación con dos marcas de 400 mg de ibuprofeno usados para aliviar dolor musculo-esquelético. Palabras clave: Equivalencia terapéutica. Resultados del tratamiento. Ibuprofeno. Nigeria. |
Lucky L. NWIDU. MSc, FPCPharm. Lecturer, Department of Pharmacology and Toxicology. Faculty of Pharmacy, Niger Delta University. Amassoma (Nigeria).
Joshua F. ENIOJUKAN. PhD. Professor. Department of Clinical Pharmacy & Pharmacy Practice. Faculty of Pharmacy, Niger Delta University. Amassoma (Nigeria).
Azuka C. OPARAH. PhD. Senior Lecturer. Department of Clinical Pharmacy & Pharmacy Practice. Faculty of Pharmacy, University of Benin. Benin (Nigeria).
Received (first version): 26-Mar-2008
Accepted: 4-Jul-2008
INTRODUCTION
Neuromuscular neuralgia is a common complaint and reason for frequent visits to pharmacies and other health care outlets among artisans in Niger Delta Area, Nigeria. The strenuous work among these artisans coupled with deltaic nature of the environment further exacerbates the pain state. These artisans visit the pharmacies to refill their prescriptions or self medicate. The impact of pain on the society is quite enormous.1,2 Pain is not only associated with discomfort, but can also interfere with sleep, decrease quality of life and produce an increase in the psychosocial stress in affected individuals.2-4
Chronic pain has been associated with diminished quality of life and its sub-optimal treatment is a major health problem. Chronic pain has also been implicated as a constant factor in depression, social isolation, sleep disturbances and impaired ambulation.2
Patients have realized from experience over the years that not all branded or generic medicines that are pharmaceutically equivalent have similar efficacy. Also, not all brands of ibuprofen 400 mg have equivalent anti-inflammatory, antipyretic and analgesic potentials.3 This may explain why some patients are loyal to a particular brand of Ibuprofen 400 mg and refuse other brands in spite of their reported pharmaceutical equivalence. This situation poses a challenge to the community pharmacist and may also present a frustrating experience to those patients who cannot refill their prescriptions with a brand of choice. However, such preferences for generics or brand named pharmaceuticals have been shown to improve adherence in chronic conditions.5
Several studies provide evidence that various non-therapeutic brand-name and generic drugs that are pharmaceutical equivalent produce different therapeutic effects.6-10 Approximately 20% of primary care patients experience chronic pain, and pain screening is intended to improve the quality of pain management by systematically identifying patients with pain in clinical settings, but currently there is no commonly accepted gold standard for clinically important pain.11 The practice of universal pain screening has become widespread despite a lack of published research evaluating the accuracy and effectiveness of pain screening strategies. The most commonly used measure for pain screening may have only modest accuracy for identifying patients with clinically important pain in primary care.11
Beliefs and attitudes to pain are of crucial importance and various instruments for the assessment of pain are available and widely used in the literature.12-18 McGill Pain Questionnaire has been employed to establish the effectiveness and safety of Divalproex® sodium in the management of post-herpetic neuralgia.19 Katzer (2005) evaluated the clinical trial factors in pain transition models and observed that the transition coefficients fitted to various analgesics, comparing their relative efficacy, gave ibuprofen a higher efficacy than other analgesics investigated.1 That was a novel study using McGill pain questionnaire in comparing the therapeutic efficacy of two brands of the same strength of ibuprofen.
We followed up an observed trend. Artisans visiting community pharmacies in the Delta area of Nigeria would request for a specific brand of ibuprofen for pain alleviation. This study was therefore designed with the objective to investigate patients reported differences in outcome following therapy with two brands of ibuprofen 400 mg.
METHODS
This study employed a randomized, non-blinded design. Participants had musculoskeletal pain and required regular medication after long hours of manual labor. They were recruited after ethical approval and informed consent. Selected patients who met the inclusion criteria were randomly assigned into either of the two groups through a simple ballot method.
This investigation was conducted from June 2005 to June 2006 in Amassoma, Bayelsa State, Nigeria. Building construction sites where artisans in the area inhabited were used.
One hundred patients with musculoskeletal pain were recruited. A 6- point Likert scale was used to evaluate eligibility for admission into the study. We performed a linguistic validation of the instrument and piloted it on a sample of five. Some terms were consequently prefaced with local expressions. The attitudes of the participants with respect to: need of ibuprofen 400 mg, symptoms consistent with musculoskeletal pains, and the impact of pain on quality of life were evaluated.
The patients with a score of 3 and above on the 6-point Likert scale that fulfilled the following criteria were eligible for enrollment in this study: consistent musculoskeletal pains of not more than ten years, males of 18 to 60 years old, myalgia, malaise and aches and tiredness, muscle weakness and informed consent to participate in the study. Such participants could also understand spoken or written expressions in English Language.
Patients having peptic ulcer disease or who were using other analgesic medications; who had other pains such as chronic juvenile arthritis or rheumatic pains; those using antihypertensive medications such as furosemide or thiazide diuretic were excluded. Lack of informed consent and language barriers were also exclusion factors.
Data collection was based on the Modified Short-Form of McGill Pain Questionnaire (SF-MPQ), Visual Analogue Scale (VAS), Present Pain Intensity (PPI) scale, and Clinical Global Impression of Improvement (CGII) scale.
The McGill Pain Questionnaire had been developed from a theoretical consideration of three separate components of the experience of pain namely sensation of pain, its emotional effects and the cognitive assessment made by pain sufferers. The instrument is one of the most extensively tested measures and has become a gold standard for assessing other measures of pain. In the McGill Pain Questionnaire, patients are presented with groups of 80 adjectives and are required to select one from each group that most closely matches with their own pain. The weighted scores are summed to produce a total. The SF-MPQ consists of 15 selected adjectives anchored on a four-point response scale. In this scale, an estimated summation of the weighted means from each group of adjectives and the mean of the total index for each group as well as the standard deviation were computed.
In the Present Pain Intensity (PPI) scale, patients are required to select one out of the six words used to describe the intensity of pain: no pain=0, mild=1, discomforting=2, distressing=3, horrible=4, and excruciating=5. The PPI was pre-tested and post-tested. Calculation was based on weighted mean with standard deviation for five patients.
The Visual Analogue Scale requires patients to imagine their extent of pain on a numerical axis that ranges from 0 (no pain) to 100 (worst pain imaginable).
Clinical Global Impression of Improvement questionnaire allows patients to compare the changes in their illness over time and rate them as follows: very much worse=1, much worse=2, minimally worse=3, no change=4, minimally improved=5, much improved=6, and very much improved=7. Weighted means are computed and compared.
Again, an attempt was made to produce a linguistic validation of the survey instruments using a sample of 5 participants in the pilot study.
The potency of the two selected brands of ibuprofen 400 mg was determined in a pharmaceutical analysis. The participants were randomly divided into two groups and placed on each brand of Ibuprofen 400 mg (A or B) twice daily for three (3) days for assessment of musculoskeletal pain alleviation. Forty-two (42) patients received brand A while 43 received brand B of ibuprofen 400 mg.
Data were collected through personal interview and consultation with patients at the beginning of the study when participants were assisted to fill the pretreatment McGill Pain Questionnaire. The same was done post-treatment with the post-treatment questionnaires. The participants were visited every evening and reinforced to keep to the study protocol.
The interval data obtained from the McGill pain questionnaire and Clinical Global Impression of Improvement questionnaire were analyzed by calculating the mean, standard deviation. Interval data from both groups were compared using the Students t-test with the aid of GraphPad Instat version 2.05a at 95% confidence interval.
RESULTS
Of the 100 persons approached for the study, 85 agreed to participate, giving a response rate of 85%. Fifteen were excluded because 12 took other drugs and 3 withdrew their consent. There were 42 participants, mean age 29.2 (SD=1.37) assigned to brand A and 43 (mean age=28.8 SD=1.14) in brand B. There was no significant difference between the variables that described the two groups, 61.9% and 69.8% of brand A and brand B participants respectively had experienced musculoskeletal pains for duration of one year and below.
Pharmaceutical assay of both brands indicated that brand A contained 100.3% (SD=0.2) w/w of ibuprofen with a dissolution profile of 92.2% (SD=0.6) in 60 minutes while brand B contained 98.2% (SD=0.7) w/w of ibuprofen with dissolution profile of 86.8% (SD=0.3) in 60 minutes.
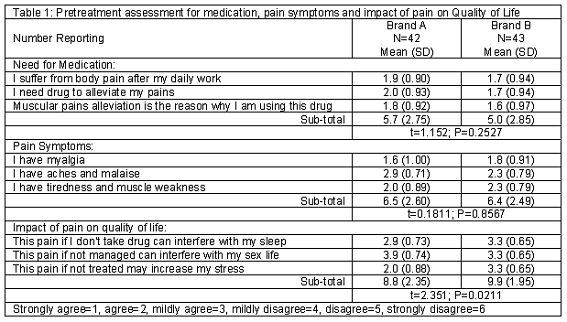
Table 1 compares participants that received brands A and B of ibuprofen 400 mg in terms of their need for medication to alleviate pain symptoms, and the impact of pain on their quality of life. Forty-two patients received brand A while 43 received brand B. Summated scores for need for medication were found to be 5.7 (SD=2.75) (brand A) and 5.0 (SD=2.85) (brand B); t=1.152, P=0.253. Pain symptoms for both groups were also comparable (6.5 SD=2.60 versus 6.4 SD=2.49; t=0.181, P=0.857). Brand B participants however reported a significantly higher impact of pain on their quality of life: 8.8 (SD=2.35) and 9.9 (SD=1.95); t=2.351, P=0.021.
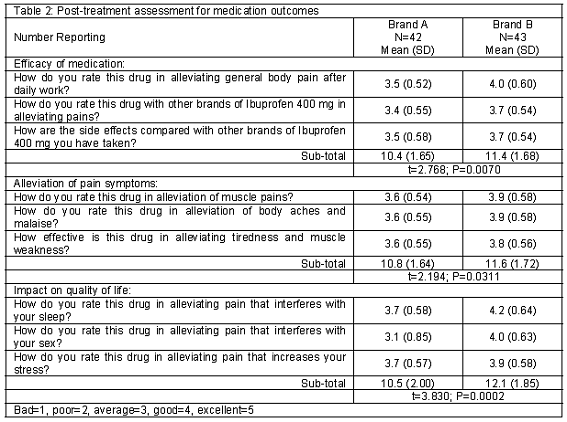
Post-treatment assessments of medication outcomes are reported in Table 2. Brand B was consistently rated higher than brand A. Scores for medication efficacy were 10.4 (SD=1.65) (brand A) and 11.4 (SD=1.68) (brand B); t=2.768, P=0.007. Alleviation of pain symptoms: 10.8 (SD=1.64) and 11.6 (SD=1.72); t=2.194, P=0.031. Similarly, rated scores on the impact of pain on quality of life were 10.5 (SD=2.00) and 12.1 (SD=1.85); t=3.830, P<0.001. However, reported pharmacokinetic profiles of brands A and B were statistically comparable: 3.7 (SD=0.60) and 3.9 (SD=0.59); t=1.549, P=0.1251, Table 3.
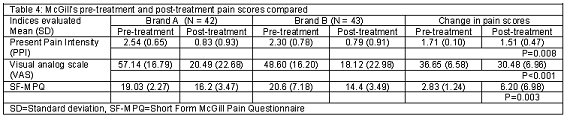
Table 4 shows the mean and standard deviation of the pain scores before and after administration of the two brands of ibuprofen 400 mg in the two groups of participants. The difference in pretreatment and pos-treatment pain scores was significant (P<0.05). There was a reduction in Present Pain Intensity scores by 32.7% and 34.3% for Brand A and brand B participants respectively. The decrease in Visual Analog pain score was 35.9% and 37.3% for brand A and brand B participants respectively. The decrease in SF-MPQ was by 85.1% and 69.9% for the brand A and brand B groups respectively.
The post -treatment evaluation of health status with respect to symptoms alleviation; each symptom alleviation was assessed as 50.0% for myalgia, 42.9% for aches and malaise, and 38.1% for tiredness and weakness for the brand A participants and 55.8%, 67.4% and 62.8% respectively for the brand B participants.
The clinical global impression of improvement for both groups of patients indicated an improvement rate of 71.4% and 61.9% for brand A and 81.4% and 74.4% for brand B participants with respect to pain and symptoms improvement respectively. The impression of improvement with respect to quality of life in three domains of sleep, sex and stress were 64.3%, 19.0% and 59.5% for brand A, and 60.5%, 48.8% and 39.5% for brand B participants respectively.
DISCUSSION
Pre-treatment evaluation revealed that both groups of participants experienced nociceptive and neuropathic pains. These enrollees therefore required medication to alleviate their symptoms complex, which could negatively impact on their quality of life regarding sleep, sex and stressful pains.
Though both brands met the potency requirement for comparison, the in vitro release profile of brand A was slightly better than Brand B. However, this did not reflect in the patient reported outcome. Brand B was observed to be more efficacious than brand A. This may be partly due to differences in bioavailability between the two brands. Studies have demonstrated that drugs known to be legally chemically equivalent are not at the same time clinically equivalent.6-9
All the symptoms complex of musculoskeletal pains, myalgia, aches and malaise, tiredness and weakness responded more favorably to brand B than brand A of ibuprofen. Furthermore, the patient reported pharmacokinetic parameters such as: onset of action, duration of action as well as the degree of relief recorded for brand B showed efficacy better than brand A, though the difference was not statistically significant.
Pretreatment and post-treatment pain scores using McGill Pain evaluation showed that brand B scored greater decrease in pain intensity and increase in visualization of pain relief and therapeutic effectiveness than brand A in both groups of participants with little incidence of adverse drug reactions. However, the overall pain-rating index was more for brand A than Brand B. This reflects the subjective nature of pain and the instruments used for evaluation.
Health status20 was greatly improved on all the parameters assessed for brand B more than brand A, thus confirming the reason for greater inter-individual preference of this brand in pharmacies and other retail outlets. Alleviation of pains and its associated symptoms complex were more with brand B. Though pain impacting on the sex domain of the quality of life status was minimally relieved. The results showed that brand B marginally had a better therapeutic efficacy than brand A with respect to improvement of health status.
On the clinical global impression of improvement (CGII), greater impression of improvement was noted with brand B participants than brand A.
This study supports that the clinical reported outcome does not always correlate with in vitro release profile. This reiterates the need for bioequivalence study on all brands of a medication before they are approved for human consumption and included in the formulary. Pharmacists working in primary health care centers such as community pharmacies can give a good feedback on the clinical effectiveness based on patients reported outcome about the various brands of medicines they receive. Widespread public health education and collaboration between providers and consumers of health services will also help in getting useful feedback that can promote reformulation of existing brands so that there can be improvement in clinical/therapeutic efficacy and safety.
Limitations of this study include the subjective nature of pain and the instruments used in the evaluation. Most commonly used pain instruments have been reported to exhibit moderate accuracy in identifying clinical cases.11 Potential selection bias, previous experience, familiarity with the brands of ibuprofen and direct to consumer advertising of the medication would affect therapy outcomes.
Though an attempt was made to produce a linguistic validation of the survey instruments, the effect of cultural and language barriers cannot be ruled out. Despite these limitations, the study provides an insight into how patients reported outcome might be explored to provide a feedback in community pharmacy practice.
CONCLUSIONS
This clinical study infers that though the two brands of ibuprofen 400 mg are legally pharmaceutical equivalent, they are not clinically equivalent. In most of the parameters evaluated, brand B was rated more efficacious than brand A. This explains the patients preferences and demand for this brand of ibuprofen in the Nigerian community.
CONFLICT OF INTEREST
None declared.
| References |
1. Katzer M. Pain alleviation Ibuprofen, 2005. Available at htpp://72.14.203.104/search?q=cahe:Z_hfj01Y1Z1J:www.informs-cs.org/wsc05papers/280.pdf+pain+alleviation+with+ibuprofen (Accessed 14/06/06). [ Links ]
2. Main CJ, Williams AC. ABC of Psychological Medicine, Musculoskeletal Pain. BMJ 2002;325:534-537. [ Links ]
3. US Pharmacist, Low Back Pain: Patient Management: Available at http://www.USpharmacist.com/index.asp?page=ce/105253/default.htm (Accessed 14/04/06). [ Links ]
4. Kim CS. (2002). Musculoskeletal Pain in Adolescents. Available at Http/www.Postgradmed.com/issues/2002/04-02/km.htm (Accessed 04/06/2006). [ Links ]
5. Shrank WH, Hoang T, Ettner SL, Glassman PA, Nair K, DeLapp D, Dirstine J, Avorn J, Asch SM. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med 2006;166:332-337. [ Links ]
6. Meredith P. Bioequivalence and other unresolved issues in generic substitutions. Clin Therap. 2003;25(11):2875-2890. [ Links ]
7. Sharoky M, Perkal M, Tabatznik B, Cane RCJ, Costello K, Goodwin P Comparative efficacy and bioequivalence of a brand-name and a generic triamterene-hydrochlorothiazide combination product. Clin Pharm. 1989;8:496-500. [ Links ]
8. Eldon MA, Kinkel AW, Daniel JE, Latts JR. Bioavailability of propranolol hydrochloride tablet formulations: application of multiple dose crossover studies. Biopharm Drug Dispos. 1989; 10:69-76. [ Links ]
9. Gleiter CH, Gundert-Remy U. Bioinequivalence and drug toxicity. How great is the problem and what can be done? Drug Safety. 1994 11:1-6. [ Links ]
10. White A (1998). Measuring pain. Available at http://www.medical-acupuncture.co.uk/journal/1998nov/six.shtml (Accessed 04/06/2006). [ Links ]
11. Laurie Barclay L, Lie D. Pain numeric rating scale may be only moderately accurate for pain screening. J Gen Intern Med. Published online August 1, 2007. Available at: http://www.medscape.com/viewarticle/563080 (Accessed 26/03/2008) [ Links ]
12. McDowell I, Newell C. Measuring Health; a guide to rating Scales and questionnaires. Oxford University Press, 1996 New York. [ Links ]
13. Vincent CA, Chapman CR. Pain measurement and the assessment of acupuncture treatment. Acupuncture in Medicine, 1989 6(1):14-19. [ Links ]
14. Galluzzi KE (2006). Management of Neuropathic Pains Available at http://www.jaoa.org/cgi/content/full/105/suppl_4/S12 (Accessed 14/06/2006) [ Links ]
15. Melzack R. The McGill Pain Questionnaire. Pain 1975; 30:191-197. [ Links ]
16. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 1983;17:45-56. [ Links ]
17. Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophilising Scale. Development and Validation. Psychological Assessment. 1995;7(4):524. [ Links ]
18. Galler BS, Jessen MP. Development and preliminary validation of pain measures specific for neuropathic pains; the Neuropathic Pain Scale. Neurology. 1997;48:332-338. [ Links ]
19. Kochar DK, Garg P, Bumb RA, Kochar SK, Mehta RD, Beniwal R, Rawat N. Divalproex sodium in the management of post-herpetic neuralgia: a randomized double-blind placebo-controlled study. QJM. 2005;98(1):29-34. [ Links ]
20. Hunt SM, McKenna SP, McEwen J, Williams J, Papp E. The Nottingham Health Profile: subjective health status and medical consultations. Soc Sci Med [A]. 1981 May;15(3 Pt 1):221-229. [ Links ]