My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Pharmacy Practice (Granada)
On-line version ISSN 1886-3655Print version ISSN 1885-642X
Pharmacy Pract (Granada) vol.7 n.1 Redondela Jan./Mar. 2009
| Original Research |
Relationship between drug interactions and drug-related negative clinical outcomes
Relación entre interacciones medicamentosas y resultados clínicos negativos de la medicación
Javier CREMADES, Mario GONZALO, Isabel ARREBOLA.
| ABSTRACT Drug interactions may represent an iatrogenic risk that should be controlled in community pharmacies at the dispensing level. Key words: Drug Interactions. Community Pharmacy Services. Spain. | RESUMEN Las interacciones medicamentosas pueden representar un riesgo iatrogénico que debería controlarse en las farmacias comunitarias durante la dispensación. Palabras clave: Interacciones medicamentosas. Servicios de farmacias comunitarias. España. |
Javier CREMADES. PhD. Community Pharmacists at Aspe, Alicante (Spain).
Mario GONZALO. BScPharm. Community Pharmacist at Crevillente, Alicante (Spain).
Isabel ARREBOLA. BScPharm. Community Pharmacists at Aspe, Alicante (Spain).
Received (frst version): 23-Jan-2008
Accepted: 5-Feb-2009
INTRODUCTION
A drug interaction is defined as the alteration of the effect of one drug by the presence of another. The number of drug interactions increases with to the number of drugs used. Because increased life expectancy is accompanied by an increase in medication use, this field of pharmacotherapy is an important public health issue.1,2
The incidence of drug interactions is a controversial issue. Study results vary greatly because of population type and methodology. Apart from this complexity, we must distinguish between potential drug-drug interactions (potential DDI) and drug-drug interactions that actually occur.2
The frequency of potentially dangerous interactions in ambulatory patients is genuinely low.3,4 However, if we take into account the large number of drugs that are prescribed and dispensed on a daily basis, a considerable number of patients can potentially be at risk.2 Even though few cases of serious interactions occur, the suspected iatrogenic risks must be considered and controlled.4,5 Accordingly, an important percentage of hospital admissions caused by medication can be attributed to drug interactions.6-11
The European Councils resolution about the pharmacists role within the health security framework establishes that verifying the appropriateness in the prescription and the possible interactions is a desirable practice.12 In this context, the community pharmacy is in a privileged position to detect these phenomena because medical prescriptions from different physicians, dental prescriptions, and self-medication converge in the pharmacy.13 In Spain, one study14 shows that only 6% of pharmacies detected a relevant drug-drug interaction, while studies undertaken in the USA15 present higher values (32%).
Most pharmacies in Spain are computerized. Pharmacy management software includes a Consejo General de Colegios Oficiales de Farmacéuticos (CGCOF) database that allows the automatic detection of potential DDIs between dispensed drugs. Although useful, the low specificity of drug interaction detection means that the attention paid to the alerts issued by these computer systems is lost.4,16,17 Systematically overriding potential DDIs via configuring the alert systems can be distinguished from overriding potential DDIs generated by computer alerts without further action.18
The identification of a potential DDI does not necessarily lead to a negative outcome, as it involves characteristics of the patient, the drugs, and the use of the drugs.19 Several works have studied drug interactions and have also analyzed them according to clinical relevance.1,3,4
We analyzed the prevalence of potential DDIs detected in community pharmacies during dispensing and the relationship with negative clinical outcomes.
METHODS
The potential DDIs in connection with negative clinical outcomes were analyzed in two community pharmacies in the province of Alicante (Spain), one located in Aspe (inland) and the other based in El Campello (coastal). Both pharmacies had similar characteristics in terms of personnel and the origin of prescriptions. The study was carried out over a six-month period from June to December, 2005.
The dispensing operations data were registered according to two groups. One group (the study group) was formed by dispensing operations in which a potential DDI was detected. The second group (the comparison group) included randomized dispensing operations in which no potential DDI was detected.
Dispensing was done using commercial management software that included the CGCOF database (Spanish Pharmacists Association). This software produces an alert for potential drug interactions. The pharmacist was called by the technician when a potential DDI alert appeared. Then, the pharmacist completed the process. Potential DDIs detected by the pharmacist that were not included in this database were also registered.
All negative outcomes were classified according to the Third Consensus of Granada on Drug-related Problems and Negative Outcomes Associated with Medication.20
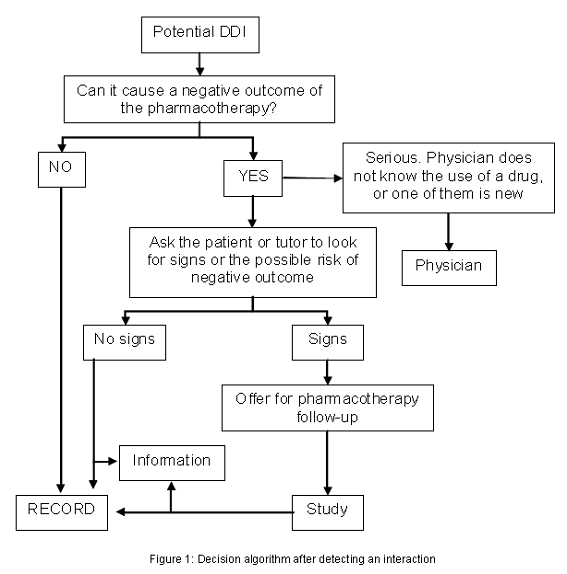
In the study group, we acted in accordance with a decision algorithm after detecting a potential DDI (Figure 1). The first criterion used in this protocol was to look at the likelihood of seeing the negative clinical outcome. The drug administration route was considered, as were the doses, and whether it involved a novel drug or they had been taken together for a long time. The case was recorded if it was considered that no negative outcome would appear.
If there was a possibility that a negative clinical outcome would appear, we then asked the patient for possible clinical signs and symptoms. If no sign was detected, when considered relevant we informed the patient to return to the community pharmacy should these signs ever appear. Conversely, when negative clinical outcome signs were detected, we offered the patient pharmacotherapy follow-up.
If a serious potential DDI was detected (defined as that which would likely require an urgent change in therapy), dispensing was suspended according to the information taken from the patient. This information had to fit at least one of two assumptions: that the physician did not know the use of all the drugs involved, and that it was the first time that the patient used one of the drugs. In both cases the patient was advised to refer back to his/her physician, and the case was excluded.
The following data were registered in the study group: the potential DDI; the patients name, age, and gender; the drugs involved and whether these drugs were prescribed; the possible negative clinical outcome; the detection of a drug-related negative clinical outcome; and any information given either to the patient or physician. In the comparison group, age and gender were also registered and expressed as the mean value and standard error (SE). The ages of both groups were compared using the Students t-test. Gender differences were evaluated between the two groups using the chi-square test. The level of statistical relevance used in both analyses was p<0.05. The rest of the data are expressed as the mean value and standard deviation (SD).
RESULTS
During this study, 39,340 dispensing operations were performed, and 417 potential DDIs were detected (1.06% of dispenses lead to a potential DDI). These 417 potential DDIs were detected in 318 dispensing operations (see Table 1). The mean rate of the potential DDIs per dispensing was 1.31 (SD=0.72 range 1 to 5). Only one (n=254 dispensing acts; 79.9%) or two (n=43 dispensing acts; 13.5%) potential DDIs were detected in the majority of cases. However, there were cases of three (n=11; 3.4%), four (n=7; 2.3%), and five (n=3 dispensing acts; 0.9%) potential DDIs registered for the same dispensing act.
The mean age of patients with potential DDIs was 59.8 (SE=0.9) years old, and it was 51.3 (SE=1.1) years old for dispensing without potential DDIs (p<0.001). Gender registration data were also significantly different between both groups, with a high rate of women in the potential DDI group (204 women of 318 dispensing acts vs. 233 women of 417 dispensing acts in the comparison group; p<0.05).
Pharmacists found 10 (2.4%) potential DDIs not appearing in the computerized alert system, despite being described in either the literature, the summary of product characteristics of the drugs involved, or in the drug description in the database.
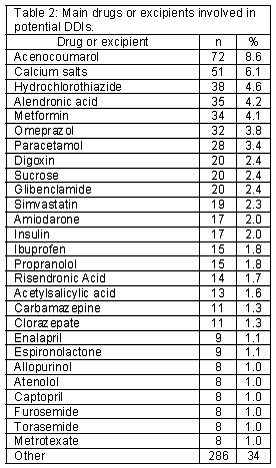
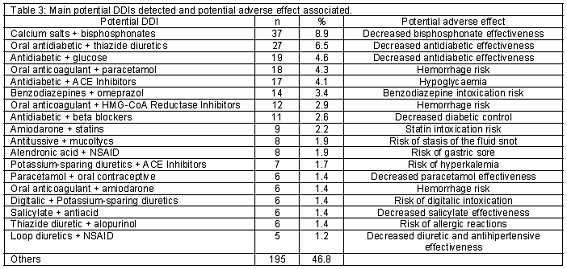
Active substances and excipients involved in the vast majority of potential DDI cases appear in Table 2. The most frequently detected potential DDIs are presented in Table 3.
In 21 (5%) potential DDIs, a dispensed drug that was not prescribed by a physician but could be obtained over the counter was detected.
Only 4 (0.96%) potential DDIs led to a negative clinical outcome (50% safety and 50% effectiveness cases). These negative outcomes involved NSAIDs in two cases and benzodiazepines in another two; 22% and 9% of the potential DDIs with NSAID and benzodiazepine, respectively, led to a negative clinical outcome.
In 23.2% of the detected potential DDI cases, the patient was directly informed about the drug interaction. This information was given to the physician in only 1% of cases.
DISCUSSION
We tested potential DDIs in connection with negative clinical outcomes in two Spanish community pharmacies. NSAIDs or benzodiazepines appeared in all potential DDIs that had negative clinical outcomes, suggesting acting with caution in potential DDIs where these drugs appear.
The frequency of potential DDIs in dispensing (1.06%) is generally lower than other studies, with prevalence from 0.8 to 80%.1,4,21-23 However, different methodologies, the potential DDIs considered, the study population, the year of the study, and inclusion of non-prescription drugs can all affect these values. For example, we only considered medications identified during a specific dispensing, and did not consider the patients medication history. DDIs must be identified to be prevented, and experience (measured as years of pharmacy training) improved the accuracy identifying potential DDIs.24 Alert systems are also effective in community pharmacies and in physician offices in reducing the number of potential DDIs.25 Nevertheless, these systems are not a panacea5,10,15,18,26, and require optimization of alerts18,26 and access to complete medication history.
The age of patients with potential DDIs was significantly higher than the group where no potential DDI was detected, consistent with other studies where the number of potential DDIs increased with the patient age1,27, as do the number of drugs used.27 We also found a higher proportion of women in the group with potential DDIs. Other studies found similar gender-related differences, suggesting that perhaps women take more medication than men, and also live longer.28 Importantly, the most frequent interaction found, bisphosphonate and calcium salts, is generally used to treat osteoporosis in postmenopausal women.
The average number of potential DDIs per patient of 1.31 (SD=0.72) was similar to that described by other authors in ambulatory patients29, but was slightly lower than in hospitalized patients.30 In this sense, Kohler et al.31 found that the number of interactions per patient was higher during hospitalization than prior to it.
Drugs usually involved in potential DDIs included some drugs frequently used in primary care, such as omeprazole or paracetamol. Other potential DDIs involved drugs that are normally used together, like calcium salts and alendronic acid. This could cause overlap in findings of other studies. For example, of the 24 drugs that caused 60% of potential DDIs, only 6 in this study were not indicated among the drugs that caused 84% of potential DDIs in Barris et al.19
In 5% of potential DDI cases, one of the drugs did not require a prescription. Strong control of potential DDIs involves the pharmacist because they might be the only health worker to evaluate the risks. In the work of Barris et al.19, these potential DDIs constituted one of the main causes of pharmaceutical intervention. These facts show the importance of a specific pharmaceutical indication and/or active dispensing of medication without a prescription.
The common potential DDIs in this study were similar to the potential DDIs found by other authors.13,18,19,22
In this study, the pharmacists detected that 0.94% of the potential DDIs led to a negative outcome in pharmacotherapy. Potential DDIs are a source of adverse drug events.7-11 NSAIDs and benzodiazepines were both involved in two drug-related negative clinical outcomes. A considerable percentage of potential DDIs with NSAID and benzodiazepine (22% and 9%, respectively) led to a negative clinical outcome. Therefore, tighter control of such cases is recommended. NSAIDs are more involved in clinically relevant pharmacological interactions.3,11
Of the interactions with a negative clinical outcome, zolpidem and diazepam did not appear in the CGCOF database, although this interaction appeared in the Summary of Product Characteristics of Stilnox (zolpidem). Nine other potential DDIs were not registered in the CGCOF database. Abarca et al.26 suggested that the performance of community pharmacy computer systems in screening potential DDIs has improved. Nevertheless, many pharmacy computer systems may be operating at low levels of sensitivity and specificity when screening for DDIs. Databases often have significant omissions and should be dynamically updated.32 Moreover, one of the ten non-registered potential DDIs led to a negative outcome, indicating the importance of both the database alerts and other combinations of drugs that may cause a potential DDI, such as combining drugs with similar or opposite effects.
Some of the drug-related negative clinical outcomes were probably not detected because six patients declined the offer of pharmacotherapy follow-up. Moreover, not detecting a sign when questioning patients does not exclude the possibility of a negative outcome associated with medication. We also only considered drugs identified during dispensing, but patients are probably exposed to a higher number of potential DDIs that could produce negative outcomes.
CONCLUSIONS
In our study, the majority of potential drug interactions have no clinical relevance, but a small proportion (0.96%) lead to negative outcomes. These negative clinical outcomes suggest acting with caution in potential DDIs where NSAIDs or benzodiazepines appear.
ACKNOWLEDGEMENTS
The authors thank Diego Echevarria, Ph.D. and Juan Miragall, M.Sc. for their critical reading of the manuscript.
CONFLICT OF INTEREST
None declared.
| References |
1. Merlo J, Liedholm H, Lindblad U, Bjorck-Linne A, Falt J, Lindberg G, Melander A. Prescriptions with potential drug interactions dispensed at Swedish pharmacies in January 1999: cross sectional study. BMJ. 2001;323(7310):427-428. [ Links ]
2. Stokley IH. Interacciones farmacológicas. Barcelona: Pharma Editores; 2004. [ Links ]
3. Calvet A, Díez de Ulzurrum, Pérez MT, Esteras J. Interacciones farmacológicas en tratamientos crónicos: medidas correctoras para su prevención en un área básica de salud. Aten Primaria. 2001;27(1):33-37. [ Links ]
4. Peng CC, Glassman PA, Marks IR, Powler C, Castiglione B, Good CB. Retrospective drug utilization review: incidence of clinically relevant potential drug-drug interactions in a large ambulatory population. J Manag Care Pharm. 2003;9(6):513-522. [ Links ]
5. Becker ML, Kallewaard M, Caspers PW, Schalekamp T, Stricker BH. Potential determinants of drug-drug interaction associated dispensing in community pharmacies. Drug Saf. 2005;28(5):371-378. [ Links ]
6. Manchon ND, Bercoff E, Lemarchand P, Chassagne P, Senant J, Bourreille J. Incidence and severity of drug interactions in the elderly: a prospective study of 639 patients. Rev Med Interne. 1989;10(6):521-525. [ Links ]
7. Huic M, Mucolic V, Vrhovac B, Francetic I, Bakran I, Giljanovic S. Adverse drug reactions resulting in hospital admission. Int J Clin Pharmacol Ther. 1994;32(12):675-682. [ Links ]
8. McDonnell PJ, Jacobs MR. Hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother. 2002;36(9):1331-1336. [ Links ]
9. Juurlink DN, Mamdani M, Kopp A, Laupacis A, Redelmeier DA. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289(13):1652-1654. [ Links ]
10. Sandson N. Drug-drug interactions: The silent epidemic. Psychiat Serv. 2005;56(1):22-24. [ Links ]
11. Becker ML, Kallewaard M, Caspers PW, Visser LE, Leufkens HG, Stricker BH. Hospitalisations and emergency department visits due to drug-drug interactions: a literature review. Pharmacoepidemiol Drug Saf. 2007;16(6):641-651. [ Links ]
12. Resolución ResAP (2001) 2 sobre el papel de los farmacéuticos en el marco de la seguridad sanitaria. Consejo de Europa. [ Links ]
13. Lien LL, Lien EJ. Preventing potential drug interactions in community pharmacie. J Clin Pharm Ther. 1994;19(6):371-379. [ Links ]
14. Acosta J, Alzaga A, Álvarez L, Gudiel M, Fernández-Llimos F. Análisis del proceso de dispensación y detección de interacción potencial en farmacias de Alcorcón (Madrid) y Bilbao. Seguim Farmacoter. 2004;2(1):32-36. [ Links ]
15. Cavuto NJ, Woosley RL, Sale M. Pharmacies and prevention of potentially fatal drug interactions. JAMA. 1996;275(14):1086-1087. [ Links ]
16. Westein MP, Herings RM, LeufkensHG, Determinants of pharmacist's interventions linked to prescription processing. Pharm World Sci. 2001;23(3):98-101. [ Links ]
17. Glassman PA, Simon B, Belperio P, Lanto A. Improving recognition of drug interactions: benefits and barriers to using automated drug alerts. Med Care. 2002;40(12):1161-1171. [ Links ]
18. Indermitte J, Beutler M, Bruppacher R, Meier CR, Hersberger KE. Management of drug-interaction alerts in community pharmacies. J Clin Pharm Ther. 2007;32(2):133-142. [ Links ]
19. Barris D, Rodríguez C, Sabio B, Garrido B. Interacciones farmacológicas detectadas en una farmacia comunitaria. Pharm Care Esp. 2003;5:261-267. [ Links ]
20. Consensus Committee. Third Consensus of Granada in Drug Related Problems (DRP) and Negative Outcomes associated with Medication (NOM). Ars Pharm. 2007;48(1):5-17. [ Links ]
21. Linnarsson R. Drug interactions in primary health care. A retrospective database study and its implications for the design of a computerized decision support system. Scand J Prim Health Care. 1993;11(3):181-186. [ Links ]
22. Doubova Dubova SV, Reyes-Morales H, Torres-Arreola Ldel P, Suárez-Ortega. Potential drug-drug and drug-disease interactions in prescriptions for ambulatory patients over 50 years of age in family medicine clinics in Mexico City. BMC Health Serv Res. 2007;7:147. [ Links ]
23. Mahmood M, Malone DC, Skrepnek GH, Abarca J, Armstrong EP, Murphy JE, Grizzle AJ, Ko Y, Woosley RL. Potential drug-drug interactions within Veterans Affairs medical centers. Am J Health Syst Pharm. 2007;64(14):1500-1505. [ Links ]
24. Weideman RA, Bernstein IH, McKinney WP. Pharmacist recognition of potential drug interactions. Am J Health Syst Pharm. 1999;56(15):1524-1529. [ Links ]
25. Halkin H, Katzir I, Kurman I, Jan J, Malkin BB. Preventing drug interactions by online prescription screening in communitypharmacies and medical practices. Clin Pharmacol Ther. 2001;69(4):260-265. [ Links ]
26. Abarca J, Colon LR, Wang VS, Malone DC, Murphy JE, Armstrong EP. Evaluation of the performance of drug-drug interaction screening software in community and hospital pharmacies. J Manag Care Pharm. 2006;12(5):383-389. [ Links ]
27. Egger SS, Rätz Bravo AE, Hess L, Schlienger RG, Krähenbühl S. Age-related differences in the prevalence of potential drug-drug interactions in ambulatory dyslipidaemic patients treated with statins. Drugs Aging. 2007;24(5):429-440. [ Links ]
28. Malone DC, Hutchins DS, Haupert H, Hansten P, Duncan B, Van Bergen RC, Solomon SL, Lipton RB. Assessment of potential drug-drug interactions with a prescription claims database. Am J Health Syst Pharm. 2005;62(19):1983-1991. [ Links ]
29. Stephens M, Kukulka G. Frequency of Drug Interactions Among Primary Care Patients at a Family Medicine Residency Clinic. Abstracts 2006 NAPCRG Annual Meeting PS184. [ Links ]
30. López Vázquez P, Rodriguez Moreno C, Duran Parrondo C, Tato Herrero F, Rodríguez López I, Lado Lado FL. Interacciones entre medicamentos prescritos al alta en un servicio de medicina interna. An Med Interna. 2005;22(2):69-75. [ Links ]
31. Kohler GI, Bode-Boger SM, Busse R, Hoopmann M, Welte T, Boger RH. Drug-drug interactions in medical patients: effects of in-hospital treatment and relation to multiple drug use. Int J Clin Pharmacol Ther. 2000;38(11):504-513. [ Links ]
32. De Diego A, Alvarellos ML, Del Barrio H. Análisis de la compleción de la base de datos del Consejo General de Colegios Oficiales de Farmacéuticos sobre interacciones entre medicamentos. Pharm Care Esp. 1999;1:184-193. [ Links ]