Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Pharmacy Practice (Granada)
versión On-line ISSN 1886-3655versión impresa ISSN 1885-642X
Pharmacy Pract (Granada) vol.7 no.4 Redondela oct./dic. 2009
| Original Research |
Risk factors of self-reported adverse drug events among Medicare enrollees before and after Medicare Part D
Factores de riesgo de eventos adversas medicamentosos autoreportados entre asegurados en Medicare antes y después de Medicare Part D
Olayinka O. SHIYANBOLA, Karen B. FARRIS, Julie M. URMIE, William R. DOUCETTE.
| ABSTRACT Objectives: Quantify risk factors for self-reported adverse drug events (ADEs) after the implementation of Medicare Part D, quantify self-reported ADEs before and after Medicare Part D and quantify the association between self-reported ADEs and increased use of prescription medication. Key words: Adverse Effects. Risk Factors. Aged. Medicare Part D. United Stated. | RESUMEN Objetivos: Cuantificar los factores de riesgo para eventos adversos medicamentosos (ADE) auto-reportados después de la implantación de Medicare Part D, cuantificar los ADE auto-reportados antes y después de Medicare Part D, y cuantificar la asociación entre ADE auto-reportados y el aumento del uso de medicamentos prescritos. Palabras clave: Efectos advesos. Factores de riesgo. Ancianos. Medicare Part D. Estados Unidos. |
Olayinka O. SHIYANBOLA. PhD. Assistant Professor. Department of Pharmacy Practice. South Dakota State University College of Pharmacy. Brookings, SD (United States).
Karen B. FARRIS. PhD. Professor. Program in Pharmaceutical Socioeconomics. University of Iowa College of Pharmacy. Iowa City, IA (United States).
Julie M. URMIE. PhD. Assistant Professor. Program in Pharmaceutical Socioeconomics. University of Iowa College of Pharmacy. Iowa City, IA (United States).
William R. DOUCETTE. PhD. Professor. Program in Pharmaceutical Socioeconomics. University of Iowa College of Pharmacy. Iowa City, IA (United States).
Received (first version): 10-Jul-2009
Accepted: 22-Oct-2009
INTRODUCTION
Adverse drug events (ADE), defined as an injury resulting from medical interventions related to the use of a drug, occur frequently in older adults.1,2 More than 90% of adults age 65 years and older use one medication per week1 and 10-25% experience an ADE.1-3 ADEs are responsible for 3.4% to 7.0% of hospital admissions4 and about 28 percent of these events are preventable.1-10 The proportion of outpatients with an ADE ranges from 5 to 35 percent, depending upon the particular definition used.4 Between 14 and 23% of older adults receive a medication they should not be prescribed8-11 and among 38 million Medicare enrollees, more than 1.9 million ADEs occur each year, 180,000 of which are life-threatening or fatal.1
One significant risk factor for having an ADE is the total number of prescribed drugs taken by older adults and the number of inappropriate medications used.7,12 Patients who take more medications and have more drug allergies are more likely to report medication symptoms, more likely to have had a prior experience of an ADE and thus more likely to be aware of the risk.3 As well, Green et al, recently showed that the number of prescribing physicians was an independent risk factor for patients self-reporting an ADE.13
In addition to medication issues, patient characteristics are associated with an increased risk of experiencing an ADE.14 A 10-year analysis of medication use showed that increased age, female gender and number of patient co-morbidities were associated with increased risk for all ADEs.15 Also, persons with more formal education believe ADEs to be significantly less severe16 while speaking a language other than English is related to patient-reported drug complication which might lead to reporting an ADE.17
In an earlier study, we examined several risk factors that were not included in previous studies, including the number of pharmacies and concern and necessity beliefs about medications. We found that number of pharmacies used in the purchase of prescription medicines, number of symptoms experienced in the past month, and concern beliefs in medicines were associated with ADEs.18 Being female and having a graduate degree also were related to reporting an ADE. There was no statistically significant relationship between number of medicines and self-reported ADEs; rather, it was concern beliefs in medicines. Necessity and concern beliefs about medicines are themes people use in the interpretation of symptoms and causal attributions related to their medicines. Necessity beliefs is related to an individual's perception of the necessity of medication for maintaining health, while concern beliefs in medicine is defined as an individual's concern about the adverse effects of medicines, based on beliefs about the potential for dependence or harmful long-term effects.19 Concern beliefs in medicines reflect patient's perceptions and experiences of specific medications. They describe patients' anxieties about the harmful effects of their prescribed medication especially, concerns about the potential adverse effects of taking it e.g. becoming too dependent on the medication or believing that regular use would lead to long term adverse effects.19,20 The concern belief scale which consists of items such as "Having to take my medicine worries me" therefore assesses the negative attitude of individual patients towards medication.19,20
The Medicare prescription drug benefit established by the 2003 Medicare Prescription Drug, Improvement and Modernization Act (MMA) was implemented to improve beneficiary access to affordable prescription medicines. However, with an increase in the number of medication used by the elderly, especially in the outpatient setting; there is an increased risk and possibility of receiving an inappropriately prescribed medicine and experiencing an ADE. A small number of studies have shown the impact of Medicare Part D on prescription drug costs and usage.21 For example, the benefit increased drug utilization via its effect on the out-of-pocket costs of the elderly and cost-related nonadherence.22-24 Among patients who had no previous drug coverage in 2005, the cost per day of supply for medications fell by 45% after the start of the benefit.25 It is estimated to have led to modest increases in prescription utilization and modest decreases in out-of-pocket expenditures.26 Despite the impact of the benefit on patients' drug utilization and expenditures, limited research has examined the effect of these changes on health outcomes. Various studies have called for the examination of the effect of the benefit on health outcomes such as ADEs.19,24
The objectives of this study were to 1) quantify risk factors for self-reported adverse drug events (ADEs) after the implementation of Medicare Part D, 2) quantify self-reported ADEs before and after Medicare Part D and 3) quantify the association between self-reported ADEs and increased use of prescription medication. The number of medications was expected to be higher in the year after Medicare Part D, and the increase in medication utilization was expected to be associated with increased reporting of ADEs.
METHODS
Design
This was a longitudinal study that included self-administered internet surveys before and after implementation of Medicare Part D. Both surveys were administered by Harris Interactive® on behalf of investigators at the University of Iowa College of Pharmacy, and the project was approved by the University of Iowa Institutional Review Board.
Patients/Setting
Harris Interactive maintains a confidential panel of individuals who will participate in telephone and/or online surveys. Harris Interactive invited individuals to participate, and they received credit from Harris Interactive for completing the survey. The inclusion criteria for the baseline and follow-up surveys were being 65 or older, English speakers, U.S. residents and enrolled in Medicare. In the baseline survey, Harris Interactive provided data to University of Iowa researchers from a convenience or non-probability sample of 1220 anonymous respondents. In the follow-up survey, Harris Interactive provided data on a sample of 1024 anonymous respondents. The follow-up survey included 436 individuals who had participated in the baseline survey and these data were linked by Harris Interactive.
Data collection
Two internet-based surveys were administered; the baseline in October 2005 and the follow-up in October of 2007. The survey was about 160 items but it included numerous skip patterns for questions that did not apply to some respondents. Also, respondents could answer part of the survey and return later if necessary for completion.
In 2005, data used in this analysis included socio-demographic data, self-rated health, number of prescription medications used, sum of symptoms experienced, concern beliefs about medicines, necessity beliefs about medicines, number of pharmacies, self-reported ADE and whether subjects skipped doses of their medications to save money or stop taking the medicines due to cost.18 Similar items were collected in the 2007 survey and were used in this analysis.
The dependent variable, self-reported ADE, was "seeing a doctor about any side effects, unwanted reaction or other problems from medicines you were taking in the past year". Previous studies have used this question in the identification of self-reported ADEs.1,29 Self-reporting ADEs represents the patients' view of this outcome, and it is important because patient reporting of possible ADEs and symptomatology is the basis for identifying ADEs in the community setting. Such reporting increases the rate at which ADEs have been identified.3
For socio-demographic data, the age of the respondent, racial background, gender, highest level of education completed, household income and geographical region/territory where respondents resided was determined. Age was recoded into three different categories. Self-rated health status was examined. Respondents rated their health and a five item response scale anchored with poor and excellent was used.27,28 To determine the number of medications used, respondents indicated the number of different prescription medicines used in the past month. Then, respondents were asked to indicate the number of medications that they took on a regular basis, among those they had taken in the past month. To examine their concerns and necessity beliefs about their medications, the 10 items from Horne et al were used.19 Five items ask about concern beliefs and five items ask about necessity beliefs. The specific concern sub-scale which assesses concern beliefs consists of the items "I sometimes worry about the long term effects of my medicines", "Having to take my medicines worries me", "I sometimes worry about becoming too dependent on my medicines", "My medicines disrupt my life", and "My medicines are a mystery to me". This scale assesses the patients' beliefs about the medication he is prescribed in relation to his concerns about taking them. Concern beliefs in medicine as a construct comprises both the emotional (e.g. having to take my medicines worries me) and cognitive representations (My medicines are a mystery to me) of patients' medication. Similar to the necessity sub-scale, the response options for the concern belief sub-scale is a five point Likert scale (ranging from strongly disagree to strongly agree) where individuals indicate their level of agreement or disagreement with each of the individual statements within each scale.19,20 Within the concern and necessity beliefs sub-scales, the five items were summed with a range of 5-25. Higher scores on the concern belief scale indicate stronger negative attitudes towards taking medicines. Previous studies using these scales reported reliability estimates ranging from 0.65-0.86 and its construct validity had been established.19
Respondents indicated the number of pharmacies where they got their prescription medicines in a typical month. They also indicated whether they had stopped taking their medications due to cost or skipped their doses to save money on a scale of never, 1-2 times, 3-4 times or more than 4 times.
Subjects were asked to indicate health symptoms they experienced in the past month (yes/no) and "past month" was used to improve recall. A list of ten symptoms to identify ADEs in primary care was included in these surveys.3 The symptoms reported by respondents included headaches, dizziness or problems with balance, stomach or gastrointestinal problems, muscle aches, incontinence or problems with urinating, rash or itching, problems with sleep, changes in mood, fatigue and sexual problems. Also, a variable "sum of symptoms" was calculated by summing responses to these ten symptoms that ranged from 0-10. The variable was categorized into 0, 1, 2, 3 and 4 or more with those having no symptoms as the comparator in the analysis. There was no opportunity to report other non-listed symptoms in 2005 and this was rectified in the 2007 survey.
Analysis
All analyses were completed by the University of Iowa investigators.
Descriptive analyses of socio-demographic and clinical/behavioral characteristics were completed. For objective one, independent factors associated with self-reporting an ADE were examined using multiple logistic regression analyses and odd ratios (ORs) with 95% confidence intervals. The dependent variable was presence of a self-reported ADE. Independent variables included socio-demographics, self-rated health, number of medications, sum of symptoms experienced, concern and necessity beliefs in medicines, number of pharmacies, and whether subjects skipped doses of their medications to save money or stopped taking the medicines due to cost. This analysis repeated the 2005 analysis previously reported18, but number of physicians was added separately.
For objective 2, the prevalence of ADE in 2007 was compared with the prevalence in 2005 for all respondents using chi-square analysis. Also, for a subset of the sample who answered both 2005 and 2007 surveys, associations between self-reported ADE and year and use of prescription medications and year were quantified using chi-square tests and paired sample t-tests, respectively.
Using only respondents who answered both surveys, logistic regression was used to determine if the number of prescription medications, concern beliefs and number of symptoms experienced differed by year and if these differences predicted self-reported ADE in 2007. These variables were included because they were statistically significant predictors in the 2005 and 2007 (reported here) analyses. Age, gender and number of pharmacies were used as control variables in the analysis. Statistical analyses were performed using SPSS software (version 15.0).
RESULTS
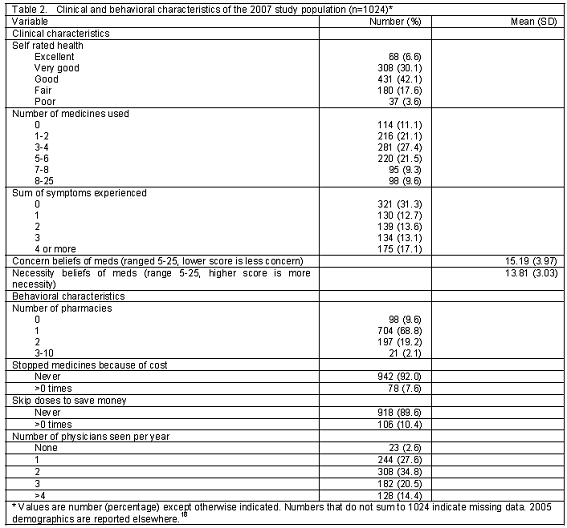
Four hundred and thirty six respondents from the baseline survey responded to the follow-up survey. New respondents were added to achieve a sample size of at least 1,000 individuals. Participants were between 65 and 98 years old, and 57.8% were female (Table 1 and 2). Most respondents were white, had some college experience, used more than one prescription medicine on a regular basis and obtained their prescription medicines from one pharmacy, had more than one regular physician and had relatively good health.
In 2007, reporting an ADE was related to concern beliefs in medicines (OR=1.09, 95%CI=1.01:1.17), 4 or more symptoms experienced (OR=2.21, 95%CI=1.23:3.98) and older age (OR=2.58, 95%CI=1.10:6.06) (Table 3). When the number of physicians seen was added, only concern beliefs (OR=1.09, 95%CI=1.01:1.17) and number of symptoms experienced (OR=1.98, 95%CI=1.09:3.62) remained statistically significant.
In 2005, eighteen percent (n=230) of all respondents reported an ADE in the last year while in 2007, 20.4% (n=208) reported an ADE (chi-square= 1.911, p=0.09). Among those who responded to both surveys (n=436), 18.4% (n=80) reported an ADE in 2005 while 24.3% (n=106) reported an ADE in 2007 (chi-square=19.98, p<0.01). There was also an increase in the mean number of prescription medicines used among those responding to both surveys: (3.83; SD=2.82) in 2005 and (4.32; SD=3.20) in 2007 (t= -5.772, p<0.01).
Among respondents who completed both surveys, reporting a self-reported ADE was related to a change in the concern beliefs in medicines (OR=1.12, 95%CI=1.05:1.19) and number of symptoms experienced in 2007 (OR=3.27, 95%CI=1.60:6.69). The mean of change in concern beliefs in medicines was 3.37 (SD=4.33) showing an increase over two years, from 11.62 (SD=3.81) in 2005 to 15.11 (SD=3.77) in 2007. A change in the number of prescription medications used was not significantly related to reporting an ADE (OR=1.04, 95%CI=0.77:1.41) (Table 4).
DISCUSSION
Over 1000 older adults currently enrolled in Medicare in 2005 and after the start of the benefit in 2007 were asked about ADEs. Self-reported ADEs increased after Medicare Part D was implemented, and the increase was associated with increased concern beliefs in medicines. Though the number of medicines used by respondents to both surveys increased, there was no association with self-reported ADEs.
Analyses in 2005 showed that ADE was related to being female, number of pharmacies used, number of symptoms experienced, concern beliefs with medicines and having a graduate degree.18 Similar significant variables were expected in the 2007 data. However, in the full model, only concern beliefs and number of symptoms experienced were significant. When the number of physicians seen regularly was added, concern beliefs and number of symptoms still remained statistically significant. Having stronger concern beliefs about medicines was significantly related to self-reporting an ADE in 2005, 2007 and over time.18 Patients with stronger concern beliefs in medicines may have self-selected to complete this survey because of its topic. This circumstance in the design does not however preclude a conclusion that concern belief in medicines is an important factor in self-reported ADEs. Respondents with stronger concern beliefs seem to be dependent on their medicines and worry about their long term effect, as indicated by the items of the scale. They may therefore be more sensitive to symptoms and pay particular attention to unwanted reactions that occur, possibly making them more likely to report an ADE.
Patients' beliefs about their medicines can be influenced by their past experiences with the medicines, adverse effects from using them, and the patient-provider communication in clinical consultations.20 Patients who have experienced a previous ADE in the past may therefore have more concerns, be more watchful for symptoms and more likely report any reactions to medicines. Also, poor provider communications about the effects, benefits and risks of prescribed medicines may shape the beliefs of the patient about their medicines and cause concerns about them. It is therefore important for self-reported assessments of ADEs by patients to be confirmed with follow-up by healthcare professionals in order to verify the causal attribution. As well, patients' beliefs about their medicines need to be assessed in clinic consultations as this may affect the interpretations of their therapy and their attribution about their medicines.
Since concern beliefs in medicines showed consistent relationships to ADEs even after Medicare Part D; these beliefs therefore appear to be an important mechanism involved in the interpretation of patients about their adverse events which may/may not be related to the start of the benefit. Due to increased access to medicines, Medicare Part D has provided opportunities for varying medication use experience which may inherently shape concerns about medicines and beliefs about their long term effects.
Among respondents who answered both surveys, there was an increase in the number of prescription medications used from 2005 to 2007. This may have occurred due to improved access to prescription drug insurance from Medicare Part D. Previous research showed that Medicare enrollees with chronic conditions such as asthma, high cholesterol, diabetes and hypertension had significant increases in the number of prescriptions on average filled per month with the start of the Part D benefit. Also, there were improvements in access to medicines in each month for these patients.25 The increase in the number of medications used may also have occurred due to the aging of the respondents across the years.
Contrary to expectation, there was no statistically significant relationship between number of medicines and self-reported ADEs in the 2005 or 2007 analyses or in the model including subjects who completed both surveys. Previous studies have shown a relationship between ADEs and number of medications, although there are conflicting results.7,10-13,30-32 Conflicting results may arise because of different ways of assessing ADEs such as using chart reviews or self-report, and the type of patients in the study, whether in-patients or outpatients, older age group or across all ages. The present finding was not consistent with previous literature using similar measures and similar population.1 This may occur because a vital socio-psychological variable such as concern beliefs in medicines was included. This variable might be more important than the number of medicines used by patients because the interpretation of symptoms and their attribution to medications may be based upon motivation to tolerate or not tolerate adverse effects, past experience with the symptoms and previous reporting about the symptom to physicians or other health professionals. Concern belief is a concept that reflects knowledge and experience from past and present medication use.
Patients who reported more symptoms were more likely to report an ADE. Patients with fewer symptoms were probably able to deal with them, while those with more symptoms would rather seek the help of health providers. Patients' interpretation of symptoms determines the causal attribution of the symptom to a medicine. It is possible that patients with more symptoms labeled the unwanted reaction as an ADE because of the increased number of medicines available due to Medicare Part D. Using more medicines may inherently change how symptoms are interpreted.
The number of prescribing physicians has been shown to be a risk factor for self-reported ADEs13, but this was not observed in our study. It is possible that concern beliefs may have accounted for the effect of this factor. Patients with stronger concern beliefs are likely to see more physicians and have more opportunities to report their ADEs. These patients may therefore have more physicians because of their concerns about their health and medicines. On the other hand, seeing more physicians regularly may increase concerns of patients about their medications and make them report ADEs.
Older age was statistically associated with a higher risk for reporting an ADE. The importance of age in predicting ADEs has been found in existent literature.5,13 Increased number of co-morbidities and regularly scheduled medications associated with advanced age may explain the effect of age.
In summary, the rates of self-reported ADE increased after Medicare Part D and there was a bivariate association between ADE and year. However, increases in medication use from 2005 to 2007 were not statistically related to the increased report of ADE. Concern beliefs and number of symptoms experienced predicted self-reported ADE among the respondents who answered both surveys and did not differ by year. These risk factors may be the driving force for self-reported ADE.
This study had some limitations. First, the measurement of ADEs was self-report and therefore may be over-estimated. Also, the subjects used in this study were online users and a convenience sample. Thus, these results are not generalizable to the whole US population 65 and older.
These findings have implications for the Medicare Part D Medication Therapy Management (MTM) programs for specified enrollees, and such programs may include face-to-face or telephonic communication with patients. This communication provides pharmacists or other MTM providers with an avenue to discuss concerns about medications with older adults and possibly decrease ADEs. In this study, ADEs increased after Medicare Part D, and these ADEs were linked to symptoms experienced due to prescription utilization and the concerns about using their medications. An MTM session provides pharmacists and other providers with an opportunity to address patients' beliefs in medicine, increase patient awareness of the correct way to take their medications through patient education and possibly decrease ADEs. Pharmacists in institutional and clinic settings have shown that MTM services can reduce ADEs.33-36 Medicare Part D provides an opportunity for similar clinical pharmacy services to be delivered in community pharmacies.
CONCLUSIONS
These findings suggest that Medicare Part D led to an increase in self-reported adverse drug events among older adults. The creation of Medicare Part D has improved drug coverage and lessened the financial burden of many beneficiaries with chronic illnesses, yet, it is important to consider if the extent of increased access and increased utilization has only improved the health and safety of elderly patients and beneficiaries. Further research is needed to more fully understand the intended effects such as utilization and costs along with its unintended consequences such as ADEs. This is vital to ensure medication safety for older adults.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
The 2005 data collection was funded by the Center for Improving Medication Prescription use in the Community at the University of Iowa College of Pharmacy, Iowa City, IA.
The 2007 survey and related work was sponsored by and the Agency for Healthcare Research and Quality (AHRQ) Centers for Education and Research on Therapeutics cooperative agreement #5 U18 HSO16094.
| References |
1. Gurwitz JH, Field TS, Harrold L, Rothchild J, Debellis K, Seger AC, Cadoret C, Fish LS, Garber L, Kelleher M, Bates DW. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003; 289:1107-1116. [ Links ]
2. Gandhi TK, Weingart SN, Borus J, Seger AC, Peterson J, Burdick E, Seger DL, Shu K, Federico F, Leape LL, Bates DW. Adverse drug events in ambulatory care. N Eng J Med. 2003;348:1556-1564. [ Links ]
3. Weingart SN, Gandhi TK, Seger AC, Seger D, Borus J, Burdick E, Leape LL, Bates DW. Patient-reported medication symptoms in primary care. Arch Intern Med. 2005;165:234-240. [ Links ]
4. Budnitz DS, Pollock DA, Wiedenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006;296:1858-1866. [ Links ]
5. Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, Laffel G, Sweitzer BJ, Shea BF, Hallisey R, Vander Vilet M, Nemeskal R, Leape LL. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274:29-34. [ Links ]
6. Institute of Medicine. Preventing medication errors. Washington DC: National academy press, 2006. [ Links ]
7. Bates DW, Boyle DL, Vander Vliet MB, Schneider J, Leape L. Relationship between medication errors and adverse drug events. J Gen Intern Med. 1995;10:199-205. [ Links ]
8. Caterino JM, Emond JA, Carmago CA. Inappropriate medication administration to the acutely ill elderly: a nationwide emergency department study, 1992-2000. J Am Geriatr Soc. 2004;52:1847-1855. [ Links ]
9. Curtis LH, Ostbye T, Sendersky V, Hutchison S, Dans PE, Wright A, Woosley RL, Schulman KA. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med. 2004;164:1621-1625. [ Links ]
10. Higashi T, Shekelle PG, Solomon DH, Knight EL, Roth C, Chang JT, Kamberg CJ, MacLean CH, Young RT, Adams J, Reuben DB, Avorn J, Wenger NS. The quality of pharmacology care for vulnerable older patients. Ann Intern Med. 2004;104:714-720. [ Links ]
11. Zhan C, Correa-de-Araujo R, Bierman AS, Snagl J, Miller MR, Wickizer SW, Stryer D. Suboptimal prescribing in elderly outpatients: potentially harmful drug-drug and drug-disease combinations. J Am Geriatr Soc. 2005;53:262-267. [ Links ]
12. Jano E, Aparasu RR. Healthcare outcomes associated with beers' criteria: a systematic review. Ann Pharmacother 2007;41:438-448. DOI 10.1345/aph.1H473. [ Links ]
13. Green JL, Hawley JN, Rask JL. Is the number of prescribing physicians an independent risk factor for adverse drug event in an elderly outpatient population?. Am J Geriatr Pharmacother. 2007;5(1):31-39. doi:10.1016/j.amjopharm.2007.03.004 [ Links ]
14. Chrischilles EA, Segar ET, Wallace RB. Self-reported adverse drug reactions and related resource use. Ann Intern Med. 1992;117:634-640. [ Links ]
15. Evans RS, Lloyd JF, Stoddard GJ, Nebeker JR, Samore M. Risk factors for adverse drug events: a 10-year analysis. Ann Pharmacother. 2005;39:1116-1118. DOI 10.1345/aph.1E642. [ Links ]
16. Dewitt JE, Sorofman BA. A model for understanding patient attribution of adverse drug reaction symptoms. Drug Inf J. 1999;33:907-920. [ Links ]
17. Liu GG, Christensen DB. The continuing challenge of inappropriate prescribing in the elderly: an update of the evidence. J Am Pharm Assoc. 2002;42:847-857. [ Links ]
18. Oladimeji OO, Farris KB, Urmie JG, Doucette WR. Risk factors for self-reported adverse drug events among Medicare enrollees. Ann Pharmacother. 2008;42;53-61. DOI 10.1345/aph.1K073 [ Links ]
19. Horne R, Weinman J, Hankins M. The Beliefs about medicines questionnaire: The development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14:1-24. [ Links ]
20. Horne R, Weinman J. Patients beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47(6):555-567. [ Links ]
21. Evans-Molina C, Regan S, Henault LE, Hylek EM, Schwartz GR. The new Medicare Part D prescription drug benefit: an estimation of its effect on prescription drug costs in a Medicare population with atrial fibrillation. J Am Geriatr Soc. 2007 Jul;55(7):1038-1043. [ Links ]
22. Lichtenberg FR, Sun SX. The impact of Medicare Part D on prescription drug use by the elderly. Health Aff (Millwood).. 2007;26(6):1735-1744. DOI 10.1377/hlthaff.26.6.1735 [ Links ]
23. Pauly M. Medicare drug coverage and moral hazard. Health Aff (Millwood). 2004;23(1):113-122. [ Links ]
24. Karaca Z, Streeter SB, Barton V, Nguyen K, Norris K. The impact of Medicare Part D on beneficiaries with type 2 diabetes/drug utilization and out-of-pocket costs. Available at http://ssrn.com/abstract=1109130 (Accessed June 7, 2008). [ Links ]
25. Neuman P, Strollo MK, Guterman S, Rogers WH, Li A, Rodday AM, Safran DG. Medicare prescription drug benefit progress report: findings from a 2006 national survey of seniors. Health Aff (Millwood). 2007;26(5):w630-643. DOI 10.1377/hlthaff.26.5.w630 [ Links ]
26. The Amundsen group, September 2007. Medicare Part D: Assessing the impact for beneficiaries without previous drug coverage and dual eligibles. Available at: www.amundsengroup.com (Accessed June 7, 2008). [ Links ]
27. Yin W, Basu A, Zhang J, Rabbani A, Meltzer D, Alexander C. The effect of the Medicare Part D prescription benefit on drug utilization and expenditures. Ann Intern Med. 2008;148:169-177. [ Links ]
28. Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. J Health Soc Behav. 1997;38(1):21-37. [ Links ]
29. Bailis DS, Segall A, Chipperfield JG. Two views of self-rated health status. Soc Sci Med. 2003;56:203-217. [ Links ]
30. Chrischilles E, Rubenstein L, Van Gilder R, Voelker M, Wright K, Wallace R. Risk factors for adverse drug events in older adults with mobility limitations in the community setting. J Am Geriatr Soc. 2007;55:29-34. [ Links ]
31. Piecoro LT, Browning SR, Prince TS, Ranz TT, Scutchfield FD. A database analysis of potentially inappropriate drug use in an elderly Medicaid population. Pharmacotherapy. 2000;20:221-228. [ Links ]
32. Hanlon JT, Schmader KE, Koronkowski MJ, Weinberger M, Landsman PB, Samsa GP, Lewis IK. Adverse drug events in high risk older outpatients. J Am Geriatr Soc. 1997;45:945-948. [ Links ]
33. Bond CA, Raehl CL, Franke T. Clinical pharmacy services, hospital pharmacy staffing, and medication errors in United States hospitals. Pharmacotherapy. 2002;22(2):134-147. [ Links ]
34. Kucukarsian SN, Peters M, Mlynarek M, Nafziger DA. Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163:2014-2018. [ Links ]
35. LaPointe NM, Jollis JG. Medication errors in hospitalized cardiovascular patients. Arch Intern Med. 2003;163:1461-1466. [ Links ]
36. Schnipper JL, Kirwin J, Cotugno MC, Wahlstrom SA, Brown BA, Tarvin E, Kachalia A, Horng M, Roy CL, McKean SC, Bates DW. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565-571. [ Links ]