My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Pharmacy Practice (Granada)
On-line version ISSN 1886-3655Print version ISSN 1885-642X
Pharmacy Pract (Granada) vol.8 n.3 Redondela Jul./Sep. 2010
ORIGINAL RESEARCH
Pattern and quality of scientific communications on drug safety produced by a regional pharmacovigilance center in Nepal
Patrón y calidad de las comunicaciones científicas sobre seguridad de medicamentos producidas por un centro regional de farmacovigilancia en Nepal
Subish Palaian1, Mohamed I. Ibrahim2, Pranaya Mishra3
1. Discipline of Social and Administrative Pharmacy, School of Pharmaceutical Sciences. Universiti Sains Malaysia. Penang, (Malaysia) and Department of Hospital and Clinical Pharmacy/ Pharmacology. Manipal Teaching Hospital/ Manipal College of Medical Sciences. Pokhara (Nepal).
2. Discipline of Social and Administrative Pharmacy, School of Pharmaceutical Sciences. Universiti Sains Malaysia. Penang, (Malaysia).
3. Department of Pharmacology. Saba University School of Medicine, Saba (Netherlands-Antilles).
SUMMARY
Analyzing the pattern and quality of scientific communications on pharmacovigilance can help the regional centers in Nepal and other developing countries to develop approaches for communicating effectively medicine safety issues. This kind of research is lacking in developing countries.
Objectives: To analyze the pattern and quality of scientific communications on drug safety produced by the regional pharmacovigilance center at western Nepal.
Methods: Various conference abstracts and journal publications produced by the center during its initial four years of establishment (14th September 2004 till 13th September 2008) were identified. These communications were categorized in to case reports, review articles, conference presentations, short communications, newsletter and bulletin articles, original research and case series. In addition, the quality of the case reports were evaluated as per International Society of Pharmacovigilance/International Society of Pharmacoepidemiology (ISoP/ISPE) guidelines on the requirements for submitting case reports on adverse event reports in biomedical journals.
Results: During the study period, 53 scientific communications were produced by the staff of the regional pharmacovigilance center in relation with drug safety. Among these, 18 (34%) were related to case reports and letters. The median (interquartile range) age of the patients described in the case reports was 46.5 (21.7-51.2) years. Among the total 18 ADRs, four were fixed drug eruptions, followed by contact dermatitis (n=2). Majority of the published case reports were related to skin (n=13; 72.2%). Antimicrobials were responsible for 27.8% (n=5) of the case reports. Among the 18 case reports published by the pharmacovigilance center, a majority followed the ISoP/ISPE guidelines. Few parameters like physical examination of the patient experiencing ADR, patient disposition, dosage and administration of the suspected drugs, and drug-reaction interface were missing in few of the cases.
Conclusion: A high percentage of the scientific communications were "case reports". A high proportion of the case reports produced by the center were of international standards. There were lacunae in "patient disposition" in few of the reports.
Key words: Adverse Drug Reaction Reporting Systems. Periodicals as Topic. Nepal.
RESUMEN
Analizar el patrón y la calidad de las comunicaciones científicas en farmacovigilancia puede ayudar a los centros regionales de Nepal y de otros países en desarrollo a desarrollar abordajes de comunicación efectiva de los problemas de seguridad de los medicamentos. Este tipo de investigación no existe en los países en desarrollo.
Objetivos: Analizar el patrón y la calidad de las comunicaciones científicas sobre seguridad de medicamentos producidas por el centro regional de farmacovigilancia de Nepal oriental.
Métodos: Se identificaron los resúmenes de conferencias y las publicaciones en revistas producidas pro el centro durante sus cuatro años iniciales desde el establecimiento (14 de septiembre de 2004 a 13 de septiembre de 2008). Estas comunicaciones fueron clasificadas en reporte de casos, artículos de revisión, presentaciones en conferencias, comunicaciones breves, newsletter y artículos de boletín, investigaciones originales y series de casos. Además, se evaluó la calidad de los reportes de casos según las guías requisitos para enviar a revistas biomédicas los reportes de casos sobre comunicaciones de eventos adversos de la International Society of Pharmacovigilance / International Society of Pharmaepidemiology (ISoP/ISPE).
Resultados: Durante el periodo de estudio, se produjeron 53 comunicaciones científicas por el personal del centro regional de farmacovigilancia sobre seguridad de medicamentos. De estas, 18 (34%) estaban relacionadas con reportes de casos y cartas. La media (rango inter-cuartilico) de edad de los pacientes descritos en los reportes de casos fue de 46,5 (21,7 - 51,2) años. Del total de 18 RAM, 4 fueron erupciones de medicamentos, seguidas de dermatitis de contacto (n=2). La mayoría de los reportes de casos publicados estaban relacionados con la piel (n=13; 72,2%). Los antimicrobianos fueron responsables por el 27,8% (n=5) de los reportes de casos. Entre los 18 reportes publicados por el centro de farmacovigilancia, la mayoría siguió las guías de la ISoP/ISPE. En unos pocos casos faltaban algunos elementos como examen físico del paciente que sufrió la RAM, disposición del paciente, dosis y administración del medicamento sospechoso, y medio de la reacción medicamentosa.
Conclusión: Un elevado porcentaje de las comunicaciones científicas eran reportes de casos. Una alta proporción de los reportes producidos por el centro seguían los estándares internacionales. Existe una laguna en "la disposición del paciente" en algunos de los reportes.
Palabras clave: Sistemas de comunicación de reacciones adversas medicamentosas. Revistas como tema. Nepal.
Introduction
In spite of several available mechanisms to ensure safe use of medicines, it is often inevitable to prevent the occurrence of harmful effects due to medicines. Many times, the effects produced by the medicines are unique and hence may not be predicted by everyone. For a better patient care, the healthcare professionals need to know about the harmful effects of the existing medicines. Similarly, the drug regulatory authority and the consumers also need to know about the drug safety issues. These information can help in early detection and prevention of similar Adverse Drug Reactions (ADRs) occurring in the future. Thus, communicating the medicine safety information is very important in any pharmacovigilance programs. Many times, even a single case report can make a major difference. For example, the case report on thalidomide causing phocomelia by the Australian obstetrician had created a huge awareness among the drug regulatory authorities and healthcare professionals worldwide.1 Similarly, there are several occasions where regulatory actions including banning of drugs were taken based on the scientific communications available on harmful effects of medicines.2 At present, the Uppsala Monitoring Center (UMC), the WHO collaborating international center for drug monitoring collects drug safety related information and disseminates throughout the member countries of the international drug monitoring program.3 The UMC follows several strategies to improve the communications among the member countries that include publication of newsletters, bulletins, and online discussion forums. The Erice Declaration on Communicating Drug Safety Information, published in 1997, provides a vision of vigorous, open, ethical, patient-centered communications on drug safety.4 Recently, the International Society of Pharmacovigilance (ISoP) and International Society of Pharmacoepidemiology (ISPE) has developed guidelines for submitting adverse event reports for publication.5 This guideline provides the minimum criteria required in a scientific publication related to individual case reports.
Even in developed countries, a poor understanding among the healthcare professionals6-8 suggested the need for better communications about the existing pharmacovigilance programs. In developing countries, there are only a limited pharmacovigilance programs in place or often there are no such program existing in the countries. The little information available on drug safety issues are often not communicated well. In Nepal, there is very little information available on medicine safety. The Department of Drug Administration (DDA), the national drug regulatory authority of Nepal publishes a quarterly bulletin, the Drug Bulletin of Nepal (DBN) that focuses on the medicine safety issues. But, the information presented are from the developed countries and hence difficult to generalize for the local population. Moreover, in Nepal there are only limited facilities for communication in terms of biomedical journals, and bulletins. Hence, there is a need to explore the possible mechanism to communicate the available information on medicine safety issues in the country.
Moreover, there are no mandatory requirements for clinical trial data from local population prior to the drug approval. Thus, institutional based pharmacovigilance programs are the key for ensuring drug safety in the country. These centers should disseminate medicine safety information obtained from the local population and should consider drug safety communications as a priority area. One of the objectives of the regional pharmacovigilance center in the western Nepal is to communicate medicine safety issues to the regulatory authority, healthcare professionals, general public and students related to healthcare programs. This center has taken several initiatives to communicate available drug safety issues. The center publishes a "drug information bulletin" and a pharmacovigilance bulletin named "vigil". These bulletins are distributed in Manipal Teaching Hospital (MTH) and the other educational and healthcare institutes in the country and carry medicine safety information.9 The members of the center also published several case reports, letters, review articles and original research reports related to drug safety information. The center also published booklet for the healthcare professionals10 and community pharmacy practitioners11 related to pharmacovigilance. All the ADR reports from the center are reported to the "VigiFlow" a web based database on drug safety maintained by the UMC.
Periodic evaluation of the drug safety communications can be beneficial in better dissemination of the existing medicine safety information. Similarly, analyzing the various modes of communications of the pharmacovigilance center can help the other regional centers in Nepal and other developing countries to develop approaches for communicating the medicine safety issues. Hence, the proposed study is being undertaken.
The study had the following objectives.
1. To study the pattern of drug safety related scientific communications produced by the regional pharmacovigilance center,
2. To analyze the pattern of adverse drug reactions published as case reports from the regional pharmacovigilance center, and
3. To evaluate the quality of the published case reports with the International Society for Pharmacoepidemiology (ISPE) / International Society of Pharmacovigilance (ISoP) guidelines.
Methods
Study type: A cross-sectional study that analyzed the scientific publications produced by the regional pharmacovigilance center for their pattern and quality.
Inclusion and exclusion criteria: All the drugs safety related publications published from the regional pharmacovigilance center during study period (14th September 2004 till 13th September 2008) were included. The manuscripts "under review" and the articles that are "in press" were excluded from the study. The drugs´ safety related publications published in the quarterly publications of the regional pharmacovigilance center, "drug information bulletin" and "vigil" was also excluded. These publications were excluded since they do not publish case reports or information on local patients.
Data source: The journal articles and conference abstracts published at local and international levels from the regional pharmacovigilance center in relation with medicine safety were the source of data.
Modality of operation: The published articles and conference abstracts from the regional pharmacovigilance center related to medicine safety during the study period were identified and analyzed. The information obtained from the published reports were analyzed for the types of publications, and the individual case reports were analyzed for various parameters that include age and sex distribution of the patients experiencing the ADRs, type of ADRs, suspected drugs, causality, severity and preventability assessments and the outcomes of the ADRs. The case reports were also compared with the ISoP/ISPE guidelines. ISOP/ISPE guidelines provide the requirements for submitting case reports on adverse event reports in biomedical journals. These guidelines have been endorsed by the ISoP and the ISPE and are freely available on the societies´ websites.
Results
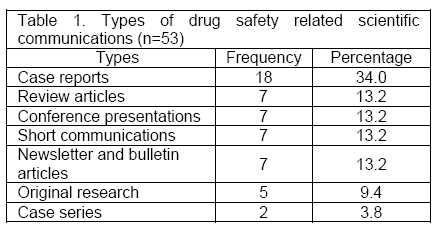
Types of drug safety related communications produced by the regional pharmacovigilance center: During the period of four years, a total of 53 communications were made by the staff of the regional pharmacovigilance center in relation with drug safety (Appendix 1). Among the 53 communications, 18 (34%) were related to case reports and letters. The details are listed in Table 1.

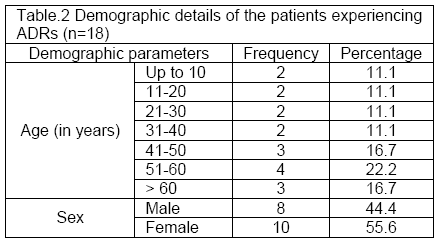
Demographic details of the patients in the published case reports: Median (interquartile range) age of the patients was 46.5 (21.7-51.2) years. The details of the demographic distribution are listed in Table 2.
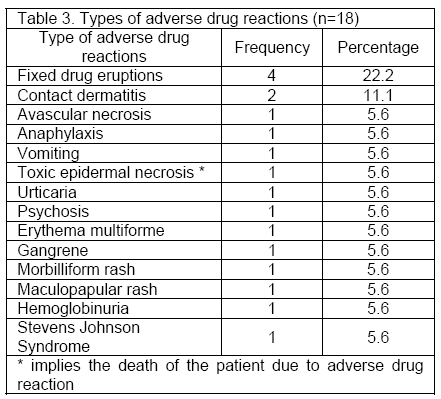
Types of adverse drug reactions published as case reports: Among the total 18 ADRs, four were Fixed Drug Eruptions (FDEs), followed by contact dermatitis (n=2). Further details are listed in Table 3.
System affected by the adverse drug reactions published as case reports: Majority of the published case reports were related to skin (n=13; 72.2%) followed by one each (5.6% each) of central nervous system, blood, gastrointestinal tract, immune system and musculoskeletal system.
Therapeutic category of the drugs implicated in the case reports: Antimicrobials were responsible for 27.8% (n=5) of the case reports. The details are listed in Table 4. The individual drugs implicated were Cefixime, Clonidine, Co-amoxiclav, Cotrimoxazole, Cyclopentolate, Dapsone, Dopamine, Erythromycin, Heparin, Ipratropium, Metronidazole, Ofloxacin, Paracetamol, Phenobarbitone, Prednisolone, Pyrazinamide, Sulfasalazine, Vitamin K.

Causality, severity and preventability assessments and outcome of the adverse drug reactions in the case reports: The causality assessment was carried out only in 17 of the 18 patients. Among the 17 ADR reports, 70.6% (n=12) were "probable", 23.5% (n=4) were definite and 5.9% (n=1) had a "possible" association with the suspected drugs. The scale used for causality assessment was Naranjo algorithm.
The severity assessment was carried out only in 13 of the 18 case reports. Out of the 13 ADR reports 46.2% (n=6) were of "moderate (Level 3)", 30.8% (n=4) were "moderate [Level 4 (b)]" and 7.7% (n=1) each of "moderate [Level 4 (a)]", "severe (Level 6)" and "severe (Level 7)". Hartwig scale was used for severity assessments in the case reports.
The preventability assessment was carried out in 10 of the total 18 case reports. Among these 10 reports, the causality in 80% (n=8) were "not preventable" and 20% (n=2) were "definitely preventable". Modified Schumock and Thornton scale was used in establishing the preventability of the ADRs.
The details of the patient outcomes were available only in 14 of the total 18 case reports. Among the 14 ADR reports, in 92.9% (n=13) of the cases, the patients conditions improved and in one case (7.1%), the patient expired due to the ADR.
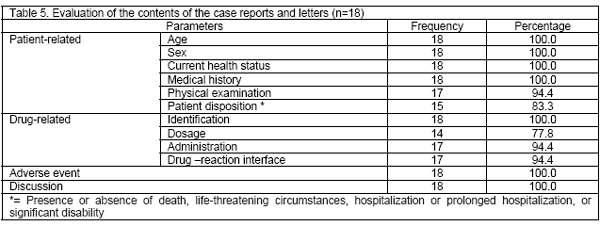
Evaluation of the contents of the case reports as per the ISoP/ISPE guidelines: The analysis of the case reports revealed that majority of the parameters recommended by ISoP/ISPE guidelines was present in most of the published case reports. There were incomplete information on physical examination of the patient experiencing ADR (94.4%), patient disposition (83.3%), dosage (77.8%) and administration (94.4%) of the suspected drugs, and drug-reaction interface (94.4%) in few of the patients. The remaining parameters were present in all the case reports. The details are mentioned in Table 5.
Discussion
Present study analyzed the pattern and quality of drug safety related communications produced by the regional pharmacovigilance center. The analysis of the publications revealed a good contribution from the regional pharmacovigilance center towards dissemination of medicine safety related issues.
During the span of four years, the center published eighteen individual reports (case report and letters) related to ADRs. The publication of single case reports, or case series, of ADRs in the medical literature is an important means of detecting new and serious reactions, particularly type-B reactions (unpredictable). The majority of first reports of ADRs still come mostly through anecdotal reports from individual doctors indicating that a patient has suffered some peculiar effect.12 In 1961, an Australian obstetrician McBride reported an increase in foetal malformations and the appearance of a rare disorder phocomelia associated with the use of thalidomide by pregnant women.1 This publication created a huge awareness among the healthcare professionals and the regulatory authorities to initiate pharmacovigilance programs in their countries. The individual case reports have also played an important role in withdrawal of harmful drugs from the market.13 However, one systematic review identified that case reports of suspected ADRs are of limited value as suspicions are seldom subjected to confirmatory investigation.14
The analysis of the case reports revealed that the most commonly implicated class of drugs to be antimicrobials accounting for 27.8% of the total cases. In a similar study from Spain that analyzed the quality of ADR reports published in certain Spanish journals, the commonly implicated drugs classes were anticoagulants and antiplatelet drugs, antibiotics, and antineoplastic agents.15 In the present study, the most common system implicated was skin. However, in the Spanish study, the system more commonly implicated were nervous system, followed by liver and skin and appendages. The higher number of case reports related to skin in this study may be because dermatological reactions are easy to identify and confirm. Moreover, the drugs commonly used vary between developing and developed countries thus making a difference in the ADR pattern.
The analysis of the case reports revealed that the case reports followed most of the criteria mentioned by the ISoP/ ISPE.5 However, in few reports the dosage and the patient disposition were not mentioned properly. In Spanish medical journals, the quality of information available on published ADR reports was low. In order to improve the quality of case reports, the Technical Committee of the Spanish Pharmacovigilance System set up the minimum information required for publication of ADR case reports in Spanish journals in the year 2004. These recommendations were found to have a better impact and approximately one third of the published case reports, following the intervention included full information. The missing data in the case reports were dose, duration of treatment as well as the ADR.15
In the present study, four case reports were published which were not documented in standard drug information sources and package insert of the particular products at the time of publication. These were FDE due to vitamin K16, contact dermatitis due to subcutaneous heparin17, vomiting due to ipratropium18, and urticaria due to clonidine.19 In Nepal as such there is no mandatory clinical trials carried out on the drugs approved. In such case, these reports play a very important role in disseminating drug safety communications.
During the study period, the center also published case series on the safety reports about pedal edema due to S-amlodipine20 and vomiting due to tramadol.21 S-amlodipine was a new drug launched for the first time in Nepal. Previous reports on this drug reported that S-amlodipine 2.5 mg is equivalent in its efficacy and tolerability when compared to amlodipine 5 mg in the treatment of mild to moderate hypertension22 and effective and well tolerated in the treatment of hypertension and is an ideal switch over therapy for patients having peripheral edema with conventional amlodipine.23 It was thus, claimed to be free of peripheral edema. However, the findings of the present study were contradicted to these findings. Similarly, though vomiting due to tramadol is well known, it is not well documented in Nepal. Researchers in this study noticed several vomiting with this drug and thus, were published.
The regional pharmacovigilance center also publishes the update from the center in the DBN, a national publication by the ministry of health, which is circulated throughout the country. It is read by health professionals from different background. This initiative might help in disseminating the drug safety information developed based on the local population.In the past the center has published on diclofenac24, paracetamol25, and cotrimoxazole.26
The major limitation of the study was that the number of scientific communications included in the study was only 53. Having more number of publications could have provided more insights on the research.
The regional pharmacovigilance center should take steps to ensure quality of publications complying with the ISoP/ISPE guidelines. Potential authors have to be sensitized on incomplete information in some important parameters of the published case reports. In developing countries like Nepal with little information on drug safety, steps have to be taken to promote the communications of existing information. The regional pharmacovigilance centers should take the responsibility on communication of medicine safety information. Similarly, the national pharmacovigilance center should also take adequate steps to promote drug safety communications.
Conclusions
The study analyzed the pattern of drug safety related publications from the regional pharmacovigilance center. High number of publications was case reports of individual patients. Most of the case reports had the basic requirements recommended by the ISoP/ISPE guidelines, thus making the quality of case reports to be "high". There were incomplete information on physical examination of the patient experiencing ADR, patient disposition, dosage and administration of the suspected drugs, and drug-reaction interface in few of the patients.
Conflict of interest
None declared.
References
1. McBride WG. Thalidomide and congenital abnormalities. Lancet. 1961;2:1358. [ Links ]
2. Khan SA, Rao PGM, Rodrigues GS, Rajan S, Heda A. From thalidomide to rofecoxib: Can we afford to wait and watch?. Int J Risk Saf Med. 2006;18:219-230. [ Links ]
3. Bate A, Lindquist M, Edwards IR, Orre R. A data mining approach for signal detection and analysis. Drug Saf. 2002;25:393-397. [ Links ]
4. Hugman B. The Erice declaration: the critical role of communication in drug safety. Drug Saf. 2006;29:91-93. [ Links ]
5. Kelly WN, Arellano FM, Barnes J, Bergman U, Edwards IR, Fernández AM, Freedman SB, Goldsmith DI, Huang K, Jones JK, McLeay R, Moore N, Stather RH, Trenque T, Troutman WG, van Puijenbroek E, Williams F, Wise RP; International Society of Pharmacoepidemiology; International Society of Pharmacovigilance. Guidelines for submitting adverse event reports for publication. Pharmacoepidemiol Drug Saf. 2007;16:581-587. [ Links ]
6. Granas AG, Buajordet M, Stenberg-Nilsen H, Harg P, Horn,A. Pharmacists' attitudes towards the reporting of suspected adverse drug reactions in Norway. Pharmacoepidemiol Drug Saf. 2007;16,429-434. [ Links ]
7. Bäckström M. Mjörndal T, Dahlqvist R, Nordkvist-Olsson T. Attitudes to reporting adverse drug reactions in northern Sweden. Eur J Clin Pharmacol. 2000;56:729-732. [ Links ]
8. Herdeiro MT, Figueiras A, Polónia J, Gestal-Otero J J. Physicians' attitudes and adverse drug reaction reporting: a case-control study in Portugal. Drug Saf .2005;28,825-833. [ Links ]
9. Shankar PR, Subish P, Mishra P. Publishing a Drug Information Bulletin: experiences from a developing country. Drug Inf J. 2008;42:555-559. [ Links ]
10. Subish P, Izham M, Mishra P. Pharmacovigilance in Nepal. A guide for healthcare professionals. Regional Pharmacovigilance Centre, Pokhara, Nepal, 2007. [ Links ]
11. Subish P, Izham M, Mishra P. Community pharmacovigilance in Nepal. A guide for community pharmacists. Regional Pharmacovigilance Centre, Pokhara, Nepal, 2008. [ Links ]
12. Lee A, Thomas SHL. Adverse drug reactions In: Walker R and Edward C. Clinical pharmacy and Therapeutics. 3rd edition Churchill Livingstone, 2003, 33-46. [ Links ]
13. Arnaiz JA, Carné X, Riba N, Codina C, Ribas J, Trilla A. The use of evidence in pharmacovigilance. Case reports as the reference source for drug withdrawals. Eur J Clin Pharmacol. 2001;57(1):89-91. [ Links ]
14. Loke YK, Price D, Derry S, Aronson JK. Case reports of suspected adverse drug reactions--systematic literature survey of follow-up. BMJ. 2006;332(7537):335-339. [ Links ]
15. Sempere E, Palop V, Bayón A, Sorando R, Martínez-Mir I. Quality of the publication of adverse drug reactions in the letters to the editor section of four Spanish internal medicine and general medicine journals. Aten Primaria. 2006;37:187-194. [ Links ]
16. Prabhu MM, Prabhu SM, Mishra P, Palaian S, Gupta S. Local eczematous allergic reaction to the menadione (vitamin k3) injection. Timisaura Med J. 2005;55(3). [ Links ]
17. Gupta S, Mishra P, Palaian S, Prabhu S, Bista D, Prabhu M. Probable cutaneous allergic response to subcutaneous heparin - a case report. Acta Dermatven APA. 2006;15(2):98-101. [ Links ]
18. Mishra P, Das RN, Prabhu M, Subish P, Upadhyay DK, Bista D. Vomiting due to inhaled Ipratropium Bromide. J Pharm Pract Res. 2005;35(2):165. [ Links ]
19. Paudel R, Kishore PV, Mishra P, Palaian S, Dwari VC. Clonidine induced acute urticarial rashes- a case report and review of literature. J Pharm Pract Res. 2006;36(3):218. [ Links ]
20. Paudel R, Palaian S, Kishore PV, Shankar PR, Mishra P. Peripheral edema due to S-amlodipine - a report of three cases. Journal of Clinical and Diagnostic Research. 2007;1(6):533-536. [ Links ]
21. Palaian S, Mishra P, Chhetri AK, Alam K. Vomiting due to tramadol: a short report from the regional pharmacovigilance center. Journal of Clinical and Diagnostic Research. 2008;2:709-711. [ Links ]
22. Pathak L, Hiremath, Kerkar PG, Manade VG. Multicentric, clinical trial of S-Amlodipine 2.5 mg versus Amlodipine 5 mg in the treatment of mild to moderate hypertension--a randomized, double-blind clinical trial. J Assoc Physicians India. 2004;52:197-202. [ Links ]
23. Anonymous. Safety and efficacy of S-amlodipine- SESA study. JAMA-India. 2003;2:81-85. [ Links ]
24. Subish P, Izham M, Mishra P. Trouble with diclofenac: a report from the regional pharmacovigilance center, Western Nepal. Drug Bulletin of Nepal. 2007;18:8-10. [ Links ]
25. Subish P, Khanal S, Izham M, Mishra P. Safety profile of Paracetamol: a report from the regional pharmacovigilance center, Western Nepal. Drug Bulletin of Nepal. 2008;19:8-10. [ Links ]
26. Subish P, Poudel A, Izham MIM, Mishra P. Dermatological manifestations of cotrimoxazole: a report from the regional pharmacovigilance center, Western Nepal. Drug Bulletin of Nepal. 2008;20:11-13. [ Links ]
Received: 22.03.10
Accepted: 26.07.10















