Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Pharmacy Practice (Granada)
versión On-line ISSN 1886-3655versión impresa ISSN 1885-642X
Pharmacy Pract (Granada) vol.8 no.4 Redondela oct./dic. 2010
ORIGINAL RESEARCH
New Zealand parent´s perceptions of the use and safety of over the counter liquid analgesics
Percepciones de los padres de Nueva Zelanda sobre el uso y la seguridad de los analgésicos líquidos sin receta
Sarah K. Bushby1, Rachel J. Anderson2, Rhiannon Braund3
1. Undergraduate Pharmacy Student, University of Otago. Dunedin (New Zealand).
2. Undergraduate Pharmacy Student, University of Otago. Dunedin (New Zealand).
3. PhD, BPharm, BSc, Senior Lecturer in Clinical Pharmacy Practice. University of Otago, School of Pharmacy. Dunedin (New Zealand).
ABSTRACT
Objective: The objective of this study was to investigate the knowledge of parents and caregivers with respect to the purchase, use and storage of liquid analgesics purchased over the counter (OTC) from pharmacies. This would enable specific strategies to be identified to increase awareness of the potential risks of these products.
Methods: Questionnaires were developed and used a combination of Likert Scales, open ended questions and yes/no answers. Randomly chosen New Zealand pharmacies (463) were asked to approach a person purchasing liquid analgesics and ask them to complete the questionnaire. Of the 105 pharmacies that participated, 96 completed parent/caregiver questionnaires were returned.
Results: When choosing a product there was a statistically significant difference between the most important factors "safety" and "active ingredient" and the least important factors "cost" and if the parent/caregiver "used it before". All parents/caregivers claim to have received verbal information from pharmacy staff, with 40% stating that they "always" receive information. The majority of parents/caregivers store medicines in a high place (n=61), in a cupboard (n=56) or a combination of these. Over half (52%) of the parents/caregivers thought that children could "never" open child resistant closures.
Conclusion: Whilst parents and caregivers choose products based on perceived safety, there is an over estimation in the perception of the protection that a child resistant closure actually offers. The general public needs to continually be vigilant in the use, storage and administration when using medication in the vicinity of children.
Key words: Child. Poisoning. Analgesics. Nonprescription Drugs. Drug Packaging. New Zealand.
RESUMEN
Objetivo: El objetivo de este estudio fue investigar el conocimiento de los padres y cuidadores en relación a la compra, uso y almacenamiento de analgésicos líquidos comprados sin receta (OTC) en farmacias. Esto podría ayudar a crear estrategias específicas identificadas para aumentar el conocimiento de los riesgos potenciales de estos productos.
Métodos: Se desarrollaron y utilizaron cuestionarios mediante una combinación de escalas Likert, preguntas abiertas y respuestas si/no. Se pidió a 463 farmacias aleatoriamente seleccionadas de Nueva Zelanda que abordasen a una persona que compraba analgésicos líquidos y le pidiese que completase el cuestionario. De las 105 farmacias que participaron, 96 devolvieron cuestionarios de padre/cuidador completos.
Resultados: Al elegir un producto, había una diferencia estadísticamente significativa en los factores más importantes "seguridad" y "principio activo" y los factores menos importantes "coste" y si el padre/cuidador "lo había usado antes". Todos los padres/cuidadores afirmaron haber recibido información oral del personal de la farmacia, afirmando el 40% que ellos "siempre" reciben información. La mayoría de los padres/cuidadores almacena medicamentos en un lugar alto (n=61), en un armario (n=56) o en una combinación de ambos. Más de la mitad (52%) de los padres/cuidadores pensaba que los niños "nunca" podrían abrir los cierres resistentes a niños.
Conclusión: Mientras que los padres y cuidadores eligen productos basados en la seguridad percibida, existe una sobre-estimación en la percepción de la protección que ofrece un cierre resistente a niños. El público en general necesita estar continuamente vigilante ante el uso, almacenamiento y administración cuando utilizan medicamentos en la inmediación de niños.
Palabras clave: Niño. Envenenamiento. Analgésicos. Medicamentos sin receta. Envases de medicamentos. New Zelanda.
Introduction
Paracetamol is a commonly used analgesic and antipyretic. The safety and efficacy of paracetamol is often taken for granted due to its status as an over-the-counter (OTC) medication.1 This is partially due to the commonly held belief that only safe medications are permitted to be sold without prescription.2 OTC medications are commonly present in homes, but often are not recognised as potent drugs that can be just as toxic as prescription drugs when taken incorrectly.3
One survey found that only 28% of parents realised that non-prescription medication could cause adverse effects.4 OTC medicines are more likely to be associated with unintentional ingestions than prescription medication, which are perceived as requiring more caution in handling and storage away from children.3 In Australia, paracetamol has been shown to be one of the most common OTC medications associated with unintentional overdose in children less than five years of age5, this is the same in New Zealand, where Poison Centre statistics show that liquid paracetamol is the agent most commonly involved in childhood accidental poisonings.6
Previous research has shown that these unintentional ingestions occur in the child´s own home in 95% of the cases and when the medication is currently in use in 75% of the cases.7 The causes of unintentional ingestion of OTC medications was investigated in Victoria, Australia and several findings included; lack of child resistant closures (CRCs), attitudes regarding the toxicity of OTC medications and lack of vigilance by parents and caregivers in the storage and administration of OTC medications.3
While there have been few studies published from New Zealand data, poisoning is in the top 10 causes of child injury (0-14) related to hospitalisation8, children under five have the highest hospitalisation rates and the most commonly implicated agents are from analgesics, antipyretics and antirheumatics.9 This is similar to other published studies in Australia10 and Kuwait.11
Few previous studies have evaluated the knowledge of caregivers which has resulted in the lack of related information in today´s scientific literature4, although one study showed that some parents may have inadequate knowledge of medications used for childhood ailments.12 In New Zealand, liquid paracetamol and ibuprofen are only available from a Pharmacy, either as a prescription item or as an OTC medication. When issued from a prescription specific dosing advice is printed for the child and has been verified by a pharmacist. When purchased from the pharmacy as an OTC product "pharmacy only", any staff member may sell them. The dosing instructions are expressed on the packaging.
When purchasing OTC products, parents´ choices have been found to be most influenced by health professionals´ recommendations, followed by cost and packaging.4 This study also found that 90% of parents and caregivers claim to always read and follow the directions on the packaging.4 One study investigating the risks in the home handling of OTC medications purchased for children found that 93% of parents believe that they store medications in an appropriate place despite storage in inappropriate places (including kitchen, bathroom and bedroom).13 They also found that parents often use inappropriate measuring devices, and that 16% of parents dose paracetamol more than four hourly. The authors concluded that there was an important role for pharmacists to educate parents regarding choice, dose, administration and storage.13 Analgesics have already been identified as a significant contributor to childhood poisonings and the liquid formulations are only available from a pharmacy. This study aimed to determine the current level of knowledge and experience of parents, and caregivers in respect to the purchase, use and storage of OTC liquid analgesics from New Zealand community pharmacies.
Methods
The questionnaire developed for parents and caregivers was adapted and modified from previous studies.4,5 The questionnaire investigated reasons for choosing a product, where OTC products are stored, and what prior knowledge parents had about accidental ingestion of medication. A combination of Likert Scales, open ended questions and yes/no answers were used.
The questionnaire was read by three pharmacy academics for clarity, face validity and to remove any ambiguities. The questionnaire was then completed by five undergraduate pharmacy students to indicate how the questions may be interpreted and answered.
Following ethical approval, questionnaires were sent to a randomly chosen half of community pharmacies in New Zealand (n=463). These pharmacies were selected from the 980 registered pharmacies within New Zealand excluding hospital pharmacies, and other non-community pharmacy registration such as manufacturers. A front of shop staff member in the pharmacy was to ask a parent or caregiver purchasing a liquid analgesic product to complete the questionnaire. Once the questionnaire was completed the respondent placed it in a postage paid envelope to be returned to the investigators. To increase the response rate, those pharmacies that completed questionnaires were entered into a draw for 2 x NZD50 and 5 x NZD20 petrol vouchers.
The raw data was entered onto a Microsoft Excel spreadsheet verbatim, and then specific coding was used to allow the data to be analyzed by SPSS. Responses to open ended questions were coded for frequency of response using keywords, while categorical data was examined for frequency. Data entry consistency was achieved by having a single data entrant, and SPSS was used to check if any data was missing. A General Linear Model consisting of ANOVA and multivariate analysis was conducted. The pairwise comparison adjustment was used for multiple comparisons (Bonferroni). The significance for all tests was set at 0.05.
Results
A total of 96 parents or caregivers completed the questionnaire, from the 105 pharmacies that returned the free post envelopes. The 96 respondents consisted of seven males and 89 females with an average age of 36 (SD=7.3, median=36, range 18-54) and an average of two (SD=0.9) children in their care.
The most commonly purchased liquid analgesic was paracetamol (71%) with the remainder purchasing ibuprofen (29%). When choosing a product the order of importance was; "safety" (mean=1.407, median=1.0), "active ingredient" (mean=1.604, median=1.0), "taste" (mean=1.934, median=2.0), "CRC closure" (mean=1.945, median=2.0), "recommended by staff" (mean=2.055, median=2.0), "previous use" (mean=2.253, median=2.0) and "cost" (mean=2.648, median 2.5). There was a statistically significant difference between the most important factors "safety" and "active ingredient" and the least important factors "cost" and if the parent/caregiver "used it before" (p<0.001).
All parents/caregivers stated that they have previously received some verbal instruction from pharmacy staff about the use of liquid analgesics with 40% stating that they "always" received information.
With respect to dosing and dosing instructions, 91% (95%CI= 90.96 to 91.06) said that they "always" read the instructions compared with 8% who either "often" or "sometimes" read the instructions. Only 86% (95%CI= 85.93 to 86.07) of parents/caregivers said that they "always" followed the dosing instructions, with 13% "often" following the instructions.
The parents/caregivers indicated that they store medicine in a high place (n=61) or in a cupboard (n=56) and other places included, bathroom, bedroom and refrigerator (Figure 1).

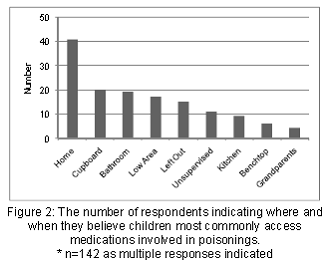
The most common response from parent/caregivers with respect to "Where/when do children most commonly access medications involved in poisoning?" was in their own home (n=41), from the cupboard (n=20), from the bathroom (n=19) and others including "low areas" "grandparents" "unsupervised" (Figure 2).
In response to the question "How often can children open child resistant closures over half (52%, 95%CI=41.9 to 52.1) of the parents/caregivers thought that children could "never" open them, 35% thought "sometimes", 9% thought "often" and 4% thought "always".
Discussion
This study has investigated parent and caregivers knowledge of OTC analgesics with respect to purchasing, storage and access. In comparison with Eiland et al.4 (2008), who found that cost was one of the most important factors for parents/caregivers when purchasing, this study found that the perceived "safety" based on the active ingredient was the most important and that cost was least important.
Reassuringly, all respondents claimed to have received advice from the pharmacy with respect to the use of liquid analgesics, however the type of information was not specified and would be worthy of further investigation. This is important as it has been shown that health professionals´ recommendations can influence parents purchasing4, and can provide advice about storage and administration.13 With respect to instructions, the findings of this study are similar to those reported in Eiland et al.4 (2008) who found that 90% of caregivers, read and follow instructions. While the possibility of "intelligent" non-compliance exists, Conroy et al. (2003) found that 16% of parents reported dosing paracetamol more than four hourly which could lead to therapeutic overdose.13
Since the introduction of child resistant closures (CRCs) there has been decreasing reports of ingestions14 and deaths.15 It is these decreases which may have led to a belief that CRCs are child-proof rather than child resistant, where 52% of the parents believed that these closures could "never" be opened by children. This in combination of a perception that OTC analgesics are "not dangerous"2-4 may make parents and caregivers complacent about the storage and handling of these medications. It was reassuring to note that the majority of parents/caregivers stored medications in a high place (n=61) or in a cupboard (n=56) or a combination of these.
Whilst there should be a strong emphasis on the safe and appropriate storage of these (and other) medications, it may be prudent to emphasize that the majority of accidental ingestions actually occur when the product is either in use, or has not yet been stored away.7 Parents and caregivers may need reminding to be more vigilant at these times, given that few parents/caregivers noted that these may occur "when left out" (n=15).
The dosing systems available on such products as liquid ibuprofen ("Nurofen for Children"®) that consist of a syringe and bung system where the liquid does not freely flow from the container even when the CRC is not on, may be an additional safety measure that might be suitable for other liquid formulations to reduce access to the product. This is an area worthy of further investigation.
Using a survey of pharmacies was chosen as these are the only places where liquid analgesics can be obtained (either via prescription or purchased over-the-counter) and so provide an opportunity to collect information from parents and caregivers directly. Potential limitations of any survey can include non-response, selection bias, recall bias and self reporting bias and so there are some limitations to this study. With respect to non-response, only 105 of the possible 463 pharmacies participated even though an incentive was offered to the pharmacy to collect this information. Given that the research team relied on the community pharmacy to request a parent or caregiver who was purchasing a liquid analgesic product we may have limited the potential response rate. This limited response rate may limit the generalisability of the findings in this study; however of the pharmacies that did participate they recruited at a rate of 91%. Additionally there may be some selection bias in the parents that were approached by the pharmacy staff, but given the random selection of the pharmacies that were asked to participate, it was anticipated that a cross-section of parents/caregivers would be included.
This study relied on self reporting which may also limit the findings. Parents may wish to say that "safety" is the most important factor, when "cost" is also one driving factor. In a previously published study investigating OTC purchases, even when a specific brand is intended, many will choose a cheaper alternative16 i.e. they may choose an active based on safety, but will purchase the cheapest version. In this study no personal details were recorded in any way so we anticipate that any social desirability bias may have been minor, also the pharmacy staff member never saw the completed questionnaire and was to give the parent/caregiver the envelope to seal. We also relied on recall of previous purchasing habits and experience which may not reflect the actual reality, although these parents/caregivers were approached during a purchase for a liquid analgesic at the time. Even with these potential limitations, the findings of this study are important in recognising the public perception of the safety, storage and use of over the counter liquid analgesics. Specific strategies to increase parents and caregivers awareness of risk associated with these products could include; specific educational campaigns to remind parents that all medications have the potential for harm if misused or taken in overdose, that child-resistant-caps are "resistant" and not "child-proof", that medications need to be stored correctly and that often childhood poisonings occur when the product is actually in use. Community pharmacy staff are ideally placed to convey such messages.
Conclusions
This study provides an insight into the current level of caregiver awareness around specific problems relevant to OTC liquid analgesic products. The general public needs to continually be vigilant in the use, storage and administration when using medication in the vicinity of children.
Acknowledgements
Dr. Simon Horsburgh (research fellow) for statistical advice and Gerald Sides (research assistant), for additional help with accuracy checks.
Conflict of interest
There are no conflicts of interest to declare.
Funding information: Funding of the studentship for SB was provided by the Child Accident Prevention Foundation of New Zealand.
References
1. Lagerlov P, Helseth S, Holager T. Childhood illnesses and the use of paracetamol (acetaminophen): a qualitative study of parents' management of common childhood illnesses. Fam Pract. 2003;20(6):717-23. [ Links ]
2. Wazaify M, Shields E, Hughes CM, McElnay JC. Societal perspectives on over-the-counter (OTC) medicines. Fam Pract. 2005;22(2):170-176. [ Links ]
3. Chien C, Marriott JL, Ashby K, Ozanne-Smith J. Unintentional ingestion of over the counter medications in children less than 5 years old. J Paediatr Child Health. 2003;39(4):264-269. [ Links ]
4. Eiland LS, Salazar ML, English TM. Caregivers' perspectives when evaluating nonprescription medication utilization in children. Clin Pediatr (Phill). 2008;47(6):578-587. [ Links ]
5. Walsh A, Edwards H, Fraser J. Over-the-counter medication use for childhood fever: a cross-sectional study of Australian parents. J Paediatr Child Health. 2007;43(9):601-606. [ Links ]
6. Safe Kids New Zealand. Unintentional childhood poisoning. http://www.safekids.org.nz/Downloads/Safekids Factsheets/Safekids Poisoning Injury Factsheet 2006.pdf. Accessed 17th November 2009. [ Links ]
7. Ozanne-Smith J, Day L, Parsons B, Tibballs J, Dobbin M. Childhood poisoning: access and prevention. J Paediatr Child Health. 2001;37(3):262-265. [ Links ]
8. Kypri K, Chalmers DJ, Langley JD, Wright CS. Child injury morbidity in New Zealand, 1987-1996. J Paediatr Child Health. 2001;37(3):227-234. [ Links ]
9. Yates KM. Accidental poisoning in New Zealand. Emerg Med (Fremantle). 2003;15(3):244-249. [ Links ]
10. Reith D, PItt W, Hockey R. Childhood poisoning in Queensland: An analysis of presentation and admission rates. J Paediatr Child Health. 2001;37:446-450. [ Links ]
11. Abahussain E, Ball D. Pharmaceutical and chemical paediatric poisoning in Kuwait: a retrospective survey. Pharm Pract (Internet). 2010;8(1):43-49. [ Links ]
12. Dawood O, Ibrahim M, Palaian S. Parent's knowledge and management of their children's ailments in Malaysia. Pharm Pract (Internet). 2010;8(2):96-102. [ Links ]
13. Conroy S, Collier J, Birchley N, Neil K, Rodgers S, McIntyre J, Choonara I, Avery A. An examination of the risk management issues in the handling at home of over-the-counter medicines purchased for children. Pharm J. 2003;271:209-213. [ Links ]
14. Assargard U, Sjoberg G. The successful introduction of child resistant closures for liquid paracetamol preparations. Saf Sci. 1995;21:87-91. [ Links ]
15. Jepsen F, Ryan M. Poisoning in children. Current Paediatrics. 2005;15:563-568. [ Links ]
16. Emmerton L. Behavioural aspects surrounding medicine purchases from pharmacies in Australia. Pharm Pract (Internet). 2008;6(3):158-164. [ Links ]
Received: 04.08.10
Accepted: 23.10.10














