Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Pharmacy Practice (Granada)
versão On-line ISSN 1886-3655versão impressa ISSN 1885-642X
Pharmacy Pract (Granada) vol.10 no.1 Redondela Jan./Mar. 2012
ORIGINAL RESEARCH
Days lost due to disability of diclofenac-induced adverse drug reactions
Días perdidos debido a discapacidad por reacciones adversas inducidas por diclofenac
Dixon Thomas1, Molly Mathew2, C. Vijaya Raghavan3, Guru P. Mohanta4, Y. Padmanabha Reddy5
1M.Pharm, M.S. Associate Professor. Raghavendra Institute of Pharmaceutical Education and Research (RIPER). Anantapur, Andhrapradesh; & Research Scholar, Karpagam University, Coimbatore, (India).
2M.Pharm, PhD. Principal, Malik Deenar College of Pharmacy, Kasaragod, Kerala (India).
3M.Pharm, PhD. Vice-Principal, PSG College of Pharmacy. Coimbatore, Tamilnadu (India).
4M.Pharm, PhD, FIC. Professor in Pharmacy. Annamalai University. Annamalai Nagar (India).
5M.Pharm, PhD, FIC. Principal, Raghavendra Institute of Pharmaceutical Education and Research (RIPER). Anantapur, Andhrapradesh, (India).
ABSTRACT
Disability Adjusted Life Years (DALY) is a widely used measure to quantify the burden of diseases or illness. DALYs for a disease is calculated as the sum of the Years of Life Lost (YLL) due to premature mortality in the population and the equivalent healthy Years Lost due to Disability (YLD). The only difference from the YLD and Days Lost due to Disability (DLD) calculation is that instead of considering the duration of Adverse Drug Reaction (ADR) in years, it is calculated in days.
Objective: DLD was measured for diclofenac tablets to prepare the ADR profile.
Methods: The study was done on the patients (18-65 years old) attending the community pharmacy at Kasaragod district, South India, with prescription of diclofenac tablets. Patients reported ADRs on their next visit to the pharmacy or they had called to the provided phone number and reported it. Disability Weight (DW) was calculated in an analogue scale from 0-1. Zero represent complete health and 1 represent death or equivalent condition. DW was multiplied with occurrence and duration of ADRs in days.
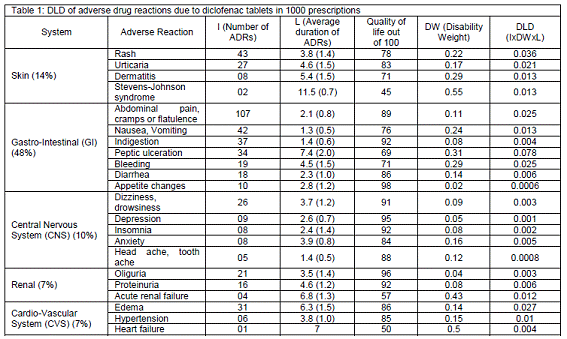
Results: About 943 patients received diclofenac tablets in 1000 prescriptions were successfully followed up for possible, probable and definite ADRs. A total of 561 reactions reported in 2010 for diclofenac tablet in the study population. There were 34 different types of ADRs under 12 physiological systems/organs. Most common reactions were on gastrointestinal (GI) system (48%), followed by skin (14%), Central Nervous System (10%), renal (7%), and cardiovascular (7%). Abdominal pain, cramps or flatulence was the highest occurring GI ADR (107), followed by 43 rashes, 42 nausea/vomiting, 37 indigestion, 34 peptic ulcers, 31 edema etc. DLD for peptic ulcer was considerably high (0.078) per 1000 of the study population on diclofenac. The most damaging ADR were peptic ulcer with or without perforation, followed by rash 0.036 DLD and edema 0.027 DLD. There was considerable DLD by acute renal failure (0.012) Steven-Johnson syndrom (0.013) even though few cases were reported.
Conclusion: Diclofenac has a complex adverse drug profile. Around 34 types of reactions were reported. Diclofenac was widely prescribed because of the experiential belief of comparative safety with other NSAIDs. The study shows the importance of pharmacovigilance even on the most prescribed medicine. Most disabling ADR for the study population was peptic ulcer with or without perforation. YLD or DLD are useful measures of calculating disability caused by ADRs. Future studies could focus on improving the usefulness & precision of DLD.
Key words: Diclofenac. Drug Toxicity. Quality-Adjusted Life Years. Disability Evaluation. India.
RESUMEN
Los años de vida ajustados a la discapacidad (DALY) es una medida ampliamente usada para cuantificar el daño de la enfermedad. Los DALY para una enfermedad se calculan por la suma de los años de vida perdidos (YLL) debido a mortalidad prematura en la población y el equivalente años de vida saludable perdidos por la discapacidad (YDL). La única diferencia en el cálculo de los YLD y los días perdidos por discapacidad (DLD) es que en lugar de considerar la duración de la reacción adversa en años, se calcula en días.
Objetivo: Se midió los DLD por el diclofenaco tabletas para preparar el perfil de RAM.
Métodos: El estudio se realizó en pacientes (18-65 años) que visitaron la farmacia comunitaria del distrito de Kasaragod, Sur-India, con una receta de diclofenac en tabletas. Los pacientes comunicaron las RAM en su posterior visita a la farmacia o llamaron o se les llamó por teléfono y las comunicaron. El peso de la discapacidad (DW) se calculó en una escala de 0-1. Cero representaba la salud completa y 1 representaba la muerte o situación equivalente. El DW se multiplicaba por la aparición y duración en días de las RAM.
Resultados: Unos 943 pacientes que recibieron diclofenac tabletas en 1000 recetas fueron seguidos con éxito para sus RAM posibles, probables y confirmadas. Durante 2010 se comunicó un total de 561 reacciones en la población en estudio. Hubo 34 tipos diferentes de RAM sobre 12 órganos o sistemas diferentes. Las reacciones más comunes fueron en el sistema gastrointestinal (48%), seguidas de piel (14%), sistema nervioso central (10%), renal (7%), y cardiovascular (7%). Dolor abdominal, calambres o flatulencia fueron las RAM GI más frecuentes, seguidas de 43 irritaciones, 42 nauseas/vómitos, 37 indigestiones, 34 úlceras pépticas, y 31 edemas. Los DLD para úlcera péptica fueron considerablemente elevados (0,078) por 1000 individuos en estudio con diclofenac. La RAM más dañina fue la úlcera péptica con o sin perforación, seguida de la irritación con 0,036 DLD y el edema con 0,027 DLD. Hubo considerables DLD por fallo renal agudo (0,012) e incluso se comunicaron algunos casos de síndrome de Steven-Johnson (0,013).
Conclusión: El diclofenac tiene un perfil de reacciones adversas complejo. Se comunicaron 34 tipos de reacciones. El diclofenac era ampliamente prescrito por la creencia empírica de la seguridad comparativa contra otros AINE. El estudio demuestra la importancia de la farmacovigilancia, incluso en los fármacos más prescritos. La RAM más incapacitante para la población de estudio fue la úlcera péptica con o sin perforación. Los YLD o los DLD son medidas útiles para calcular la incapacidad causada por RAM. Futuros estudios podrían centrarse en mejorar la utilidad y precisión de los DLD.
Palabras clave: Diclofenaco. Toxicidad de Medicamentos. Años de Vida Ajustados por Calidad de Vida. Evaluación de la Discapacidad. India.
Introduction
Diclofenac is one of the most prescribed Non-steroidal anti-inflammatory drugs (NSAID) in India or around the world.1 It is one of the main reason for higher number of Adverse Drug Reactions (ADRs) reported to diclofenac.2 So we have selected diclofenac as the representative drug to measure the ADRs of traditional NSAIDs. Adverse drug reactions of diclofenac include the reactions on skin, gastrointestinal system, central nervous system, kidney, liver, cardiovascular system, eye, mouth, musculoskeletal system, metabolic, respiratory and others. Cutaneous and gastrointestinal system ADRs are the most commonly occurring ADRs of NSAIDs.3
Cyclooxygynase-II enzyme specific NSAIDs (s-NSAIMs) were introduced to the regulatory market with the evidence of lesser adverse effects when compared to the non-specific NSAIDs (ns-NSAIDs). But further evidences suggest that the serious ADRs like cardiovascular events were higher with s-NSAIDs than ns-NSAIDs. But still there is confusion that among ns-NSAIMs which have comparable safety. There are some variations in the pharmacokinetics which contribute to the differences in safety.4,5 Empirically, diclofenac remain the drug of choice among other NSAIDs in many of the cases.
NSAIDs in general may cause 21-25% of the ADRs.6 ADR is to be considered as an illness and it induces or increases disabilities or even causes death. No medicines are free of ADRs. While considering ADR as a disease, the management strategy include prevention, treatment or mitigation.7,8
In 1990, the original Global Burden of Diseases (GBD) study was conducted by the Harvard School of Public Health in collaboration with World Bank and World Health Organization (WHO). The study was then repeated in 2002 & 2004.9 The new Global Burden Of Diseases (BOD), Injuries, and Risk Factors Study, was started on 2007 by WHO in collaboration with Harvard University, University of Washington, Johns Hopkins University and University of Queensland.10
Quality Adjusted Life Years (QALY) is used to measure the effectiveness of drugs in treating illness.11
Disability Adjusted Life Years (DALY) is used to quantify the burden of disease or illness.12 The average disability weight of peptic ulcer was counted in GBD 2004 as 0.024-0.092.13 DALYs for a disease is calculated as the sum of the Years of Life Lost (YLL) due to premature mortality in the population and the equivalent healthy Years Lost due to Disability (YLD).9
The GBD 1990 study used 3% time discounting and non-uniform age weighing. The GBD 2002 study used 3% time discounting but uniform age weighing. The GBD 2004 study used 3% time discounting & non-uniform age weighing.9
Age weighing and time discounting is indented to make more predictability to YLL. But it was widely criticized for the lack of precision.14 In the global study calculating YLL is more useful and easier than calculating YLD. YLD calculation is time consuming, it need high level expertise and resources. The incidence rates, severity of the conditions, age of onset, duration of the disease etc are required to calculate YLD.15,16 Many of the times lack of national or state wise data on diseases reduce the precision of YLD in global studies. Instead YLD is a good measure of a healthcare settings study as the simple random sampling methods can be used and limited number of professionals can complete the study in a reasonable time.17
The only difference from the YLD and Days Lost due to Disability (DLD) calculation is that instead of considering the duration of ADR in years, it is calculated in days. DLD have the advantage over Quality Of Life (QoL) because it also considers the duration and occurrence of the ADR in a particular population. So DLD is more generalizable information than QoL. It is also more valuable then DALY while measuring the health outcome due to ADRs except death. Many of the national or global studies used DALY. YLD in DALY has limited value in studies involving huge study population, but it is highly useful in regional studies involving comparatively smaller study population. This could be the one of the first study to find out DLD of diclofenac induced ADRs.
Objective: Our main objective of the study is to explain the usefulness of DLD in measuring ADRs. We had planned to measure the disability happened due to diclofenac tablets induced ADRs. DLD can be used to measure the impact of ADRs per 1000 population. And prepare the adverse drug profile of diclofenac using DLD.
Methods
DALY has been used to calculate the burden or ill health due to disease or drug related injury.15 Considering the local settings of the study, we had selected YLD, and instead of calculating it for years we calculated the disability in days.18
The study was done on the patients attending the community pharmacy at Kasaragod district, South India. Patients (18-65 years old) who were prescribed with diclofenac tablets without any Fixed Drug Combinations (FDC) were informed to report any discomfort or illness happening after consuming the drug. Patients reported ADRs on their next visit to the pharmacy or they had called to the provided phone number and reported it. The patients were enquired about the causality of the ADRs. Central Drugs Standard Control Organization (CDSCO), New Delhi, ADR reporting form was used along with Naranjo Scale for causality assessment for documenting ADRs. Possible (1-4), probable (5-8) and definite (more than 9) reactions scored by Naranjo Scale after consuming the drug were considered as diclofenac-induced ADRs.19 Doubtful adverse events and the ADRs induced by other drugs were excluded from the findings.
The formula for calculating DLD with uniform age weight and no time discounting is:
DLD = I x DW x L
I is the number of incidences of disease or injury (ADR)
DW is the disability weight and
L is the average duration of disease or injury in days up to clinical recovery or death. (In case of YLD it is years instead of days).
The disability weight value is one minus QoL out of one (QoL scored out of hundred need to be converted to one before deducting it). Disability Weight (DW) could be calculated in an analogue scale from 0-1. Zero represent complete health and 1 represent death or equivalent condition.
The average QoL at the time of ADRs are measured using the Karnofsky rating scale.20 Data collected was entered to the Microsoft Excel format and processed to find out the results. The calculation for DLD was done by using the above mentioned equation without discounting.
Results
About 943 patients received diclofenac tablets in 1000 prescriptions were successfully followed up for possible, probable and definite ADRs. Patients who received diclofenac by a separate prescription at a different occasion in 2010 were counted as a separate sample. Those who were filling the prescriptions for a particular indication of the drug were counted as the same sample. There were 561 reactions reported among the study population. The ADRs repeated in the same patient on a different occasion in 2010 or different types of ADRs observed in the same patient were counted separately. There was appreciable response in reporting any side effect suspected through the phone number provided in the dispensing packet. Average age of study population was 38 years. Among the ADRs reported, 262 causalities were males and 299 (53%) causalities were females.
Overall 34 types of ADRs are reported under 12 physiological systems/organs. Most common reactions were on gastrointestinal system (48%), followed by skin (14%), Central Nervous System (10%), renal (7%), cardiovascular (7%), Mouth (5%), Hepatic (3%), Eye (3%), Musculo-Skeletal (MS) (2%), Metabolic (0.6%), Respiratory (0.1%) and Others including Anaphylaxis (0.4%). Only 5 major groups of ADRs are mentioned in the table 1. Out of which abdominal pain, cramps or flatulence was the highest in occurrence (107). Followed by 43 with rashes, 42 nausea/vomiting, 37 indigestion, 34 peptic ulcers, and 31 edema. On the rare cases, one each of asthma & heart failure, 2 each of Stevens-Johnson syndrome & tendinitis, 3 each of jaundice and anaphylaxis reported.
Most of the times diclofenac was prescribed with anti-acid agents like ranitidine or omeprazole, still may patients developed ADRs because of noncompliance.
Eventhough Steven-Johnson syndrom is rare it scored considerable DLD (0.013) because of high disabillity weight and longer duration.
There are 107 incidence of abdominal pain and 34 incidence of peptic ulcer. Abdominal pain was the most common ADR of diclofenac. But the most damaging ADR is peptic ulcer with or without perforation. It produced 0.078 DLD which is the highest BOD among all ADRs of diclofenac. Followed by rash 0.036 DLD and edema 0.027 DLD. There was considerable DLD by acute renal failure (0.012) eventhough only 4 cases are reported.
Discussion
Disability may be caused by a disease, trauma or adverse event while therapy. BOD or DLD gives in extensive information about the severity, duration and occurrence of the disease or ADRs. DLD or YLD is a valuable tool for using locally to generate in-depth and generalizable information on disability due to disease or injury.21 YLD and DLD are good tools to measure for larger population or smaller population studies. But of course if it is a nationwide study it needs more time and high resources. The only difference from YLD and DLD is that the measure is in years and days respectively. The data is interchangeable by simple one step calculation.
There are considerably large numbers of adverse drug reactions are reported for diclofenac. Approximately half of them are in the gastrointestinal tract because of its inhibition on prostaglandins. Still empirically diclofenac is considered as one of the safest NSAID while comparing the safety profiles of other NSAIDs. This makes diclofenac a primary choice among NSAIDs.
The data developed through this observational study is highly subjective. There were very less objective data available. Subjective variation of the research staff and the study subjects could reduce the precision of results. Geographical variation is another issue which reduces the generalizability of the results to other areas of drug use. QoL is a useful but vague term. There shall be some other physical, mental, social or spiritual factors which could influence the health of the patients at the time of study. There might be high number of ADRs reported but shall not be the complete ADR profile of diclofenac. For the common ADRs some underreporting or over reporting shall not be a problem in precision but for the rare and serious ADRs even one or two missed reports would make drastic changes in DLD. Follow up studies in institutional settings would provide more complete data, but it would take more time to cover 1000 prescriptions and most of the institutional policies discourage reporting of ADRs. Another potential issue is that the average drugs in institutional prescriptions are higher than the community practice. The inpatients of the hospital also would have other health concerns which make the measurement of burden of ADRs difficult.
Conclusions
Even if diclofenac is the most chosen NSAID in the region; it has a complex adverse drug profile. The study explains the need of safety monitoring on even the most prescribed drug of choice. Large numbers of ADRs are reported in the study period. It could be correlated with higher consumption. Its adverse drug profile includes mild, moderate and severe ADRs affecting many organ systems in the body. Still it remains as the more chosen NSAID because of the experiential belief of comparative safety with other NSAIDs. YLD or DLD are useful measures of calculating disability caused by ADRs in surviving patients. Future studies shall focus on improving the usefulness and precision of DLD in measuring disability cased by ADRs.
Acknowledgment
We are expressing our gratitude to the Karpagam University research wing, Coimbatore, South India. Dr. P. Lakshmanaperumalsamy and Dr. S. Ravi were keen on proceedings of the research work. I also thank Abdul Salam & Abdul Namsheed for helping in data collection.
Conflict of interest
There are no potential conflicts of interest. This work was not intended for any commercial purpose. It was done as a part of educational activity. There were no funding agencies involved in the study. Confidentiality is maintained and there was no plan to defame any pharmaceutical product or manufacturer.
Funding information: No funds received.
References
1. Thomas D, Mathew M, Raghavan VC. Drug utilization of non-steroidal anti-inflammatory drugs at community pharmacies in South India. Int J Comun Pharm, 2010; 3(2):15-19. [ Links ]
2. Gor AP, Saksena M, Adverse drug reactions of nonsteroidal anti-inflammatory drugs in orthopedic patients. J Pharmacol Pharmacother. 2011;2(1):26-29. [ Links ]
3. Aagaard L, Hansen EH. Information about ADRs explored by pharmacovigilance approaches: a qualitative review of studies on antibiotics, SSRIS And NSAIDs. BMC Clin Pharmacol. 2009;9:4. [ Links ]
4. Fendrick AM, Greenberg BP. A Review of the benefits and risks of nonsteroidal anti-inflammatory drugs in the management of mild-to-moderate osteoarthritis, Osteopath Med Prim Care. 2009;3:1. [ Links ]
5. Thomas D, Mathew M, Raghavan VC., Risk assessment of non steroidal anti-inflammatory drugs. Available at: http://www.pharmainfo.net/reviews/risk-assessment-non-steroidal-anti-inflammatory-drugs (Accessed on Aug 2011). [ Links ]
6. Sánchez-Borges M, Caballero-Fonseca F, Capriles-Hulett A, González-Aveledo L. Hypersensitivity reactions to nonsteroidal anti-inflammatory drugs: an update, Pharmaceuticals. 2010;3:10-18. [ Links ]
7. Noel MV, Sushma M, Guido S. Cutaneous adverse drug reactions in hospitalized patients in a tertiary care center. Indian J Pharmacol. 2004;36(5):292-295. [ Links ]
8. Sharma VK, Sethuraman G, Kumar B. Cutaneous adverse drug reactions: clinical pattern and causative agents-a 6 year series from Chandigarh, India. J Postgrad Med. 2001;47(2):95-99. [ Links ]
9. WHO. The global burden of disease: 2004 Update. Geneve: WHO; 2008. [ Links ]
10. WHO. GDB study operations manual final draft, Harvard University; 2009. [ Links ]
11. NHS. NICE, Measuring effectiveness and cost effectiveness: the QALY. Available at: http://www.nice.org.uk/newsroom/features/measuringeffectivenessandcosteffectivenesstheqaly.jsp (Accessed on August 2011). [ Links ]
12. Fox-Rushby JA, Hanson K. Calculating and presenting disability adjusted life years (DALYS) in cost-effectiveness analysis. Health Policy Plan. 2001;16(3):326-331. [ Links ]
13. WHO. Global burden of disease 2004 update: disability weights for diseases and conditions. Geneve: WHO; 2008. [ Links ]
14. National Institute of Cholera and Enteric Diseases, Estimation of the burden of diarrhoeal diseases in India, Kolkata, 2004. Available at: http://www.whoindia.org/ [ Links ]
15. Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367(9524):1747-1757. [ Links ]
16. Oliveira AF, Valente JG, Leite Ida C, Schramm JM, Azevedo AS, Gadelha AM. Global burden of disease attributable to diabetes mellitus in Brazil. Cad Saude Publica. 2009;25(6):1234-1244. [ Links ]
17. Thomas D, Mathew M, Raghavan VC. Age and gender variation in burden of non-steroidal anti-inflammatory medication induced adverse drug reactions. Safety Science Monitor, 2011;15(3):4. Available at: http://ssmon.chb.kth.se/vol15/issue3/4_Thomas.pdf (Accessed on September 2011). [ Links ]
18. Lopez, A. D, Mathers CD, Ezzati M, Jamison DT, Murray CJL, Eds. Global burden of disease and risk factors. New York: Oxford University Press; 2006. http://files.dcp2.org/pdf/gbd/gbdfm.pdf (Accessed on Dec 2010). [ Links ]
19. Naranjo CA, Busto U, Sellers EM Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A Method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245. [ Links ]
20. SPNS cooperative agreement evaluation. Karnofsky Rating Scale, Version 6/11/96, 1996. [ Links ]
21. WHO. Global burden of disease database. Available at: http://www.who.int/evidence/bod (Accessed on Dec 2010). [ Links ]
Received (first version): 13.05.11
Accepted: 05.02.12