My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Pharmacy Practice (Granada)
On-line version ISSN 1886-3655Print version ISSN 1885-642X
Pharmacy Pract (Granada) vol.10 n.3 Redondela Jul./Sep. 2012
ORIGINAL RESEARCH
Disclosure and adverse effects of complementary and alternative medicine used by hospitalized patients in the North East of England
Advertencias y efectos adversos de las medicinas alternativas y complementarias usadas por pacientes hospitalizados en el noreste de Inglaterra
Nusirat Bello1, Win Winit-Watjana2, Wasim Baquir3, Kenneth McGarry4
1Department of Pharmacy, Health and Well-being (PHW), University of Sunderland, Sunderland (United Kingdom).
2Senior Lecturer in Pharmacy Practice. PHW, University of Sunderland, Sunderland (United Kingdom).
3Clinical Pharmacist. Pharmacy Academia/Research Group (PARG), Northumbria Healthcare NHS Foundation Trust, North Shields (United Kingdom).
4Senior Lecturer in Statistics for Health Sciences. PHW, University of Sunderland, Sunderland (United Kingdom).
Funding: This study formed part of PhD research that was partly funded by the University of Sunderland, but received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ABSTRACT
Objectives: This study aimed to investigate the prevalence, disclosure and adverse effects of complementary and alternative medicine (CAM) use in hospitalised patients, and to explore the associations between patients´ perceived side-effects and relevant factors.
Methods: Patients who were admitted to a district general hospital and met the eligibility criteria were interviewed using a semi-structured questionnaire. Their medications and pertinent details were verified from the medical notes. All quantitative and qualitative data were collated and analysed. A chi-squared test was performed to test the associations of the perceived CAM side-effects with the significance level determined at α=0.05.
Results: A total of 240 in-patients completed the study. They were mostly white British (98.8%). The prevalence of CAM use within two years was 74.6% and one month 37.9%. Only 19 of 91 patients (20.9%) using CAM within one month disclosed their current CAM applications. Nearly half of patients (45.8%) who used CAM within two years experienced various CAM side-effects that tended to resolve after discontinuation. Slightly more than half (57.6%) perceived CAM side-effects and their perceptions were significantly associated with gender (P=0.048) and consideration for future CAM use (P=0.033). Potential interactions between herbal remedies/dietary supplements and prescribed drugs, such as garlic with lisinopril or aspirin, were assessed in 82 patients (45.8%).
Conclusion: Most in-patients used CAM and experienced some adverse effects. The disclosure of CAM use and its adverse outcomes should be encouraged by healthcare professionals.
Key words: Complementary Therapies. Disclosure. Inpatients. United Kingdom.
RESUMEN
Objetivos: Este estudio trató de investigar la prevalencia, advertencia y efectos adversos de las medicinas alternativas y complementarias (CAM) usadas por pacientes hospitalizados, y explorar las asociaciones entre los efectos adversos percibidos por los pacientes y factores relevantes.
Métodos: Se entrevistó usando un cuestionario semi-estructurado a los pacientes que fueron admitidos en un hospital general distrital y que cumplían los criterios de elegibilidad. Se comprobaron los medicamentos y otros detalles pertinentes en la historia clínica de los pacientes. Se recogieron y analizaron todos los datos cuantitativos y cualitativos. Para comprobar las asociaciones de los efectos adversos percibidos de las CAM se realizó un test chi-cuadrado con un nivel de significación de α=0,05.
Resultados: Un total de 240 pacientes hospitalizados completó el estudio. Eran mayoritariamente británicos (98,8%). La prevalencia de uso de CAM en dos años fue del 74,6% y en un mes del 37,9%. Sólo 19 de los 91 pacientes (20,9%) que usaron CAM en el último mes reveló su uso actual. Casi la mitad de los pacientes (45,8%) que usaron CAM en los dos últimos años sufrió algunos efectos adversos de las CAM que tendieron a resolverse después de abandonarlas. Ligeramente más de la mitad (57,6%) percibieron efectos adversos de las CAM y su percepción estaba significativamente asociada con el género (P=0,048) y con la consideración sobre el futuro uso de CAM (P=0,033). En 82 pacientes (45,8%) se encontró interacciones potenciales entre plantas medicinales/suplementos dietéticos y medicamentos prescritos, tales como ajo con lisinoprilo o aspirina.
Concusión: La mayoría de los pacientes hospitalizados usó CAM y percibió algunos efectos adversos. La advertencia del uso de CAM y sus efectos adversos debería ser incentivada por los profesionales de la salud.
Palabras clave: Terapias Complementarias. Revelación. Pacientes Internos. Reino Unido.
Introduction
The public and patients have increasingly used complementary and alternative medicine (CAM) together with conventional medicine to treat or prevent some medical conditions. According to the World Health Organization (WHO), CAM that is interchangeably used with "traditional medicine" refers to "a broad set of health care practices that are not part of that country's own tradition and are not integrated into the dominant health care system".1 CAM practices2,3 exploited by patients can be generally grouped into natural products, mind-body medicine, manipulative and body-based practices, and other practices, e.g. movement therapies, qigong, etc. Some studies4,5 in the UK reported as high as 69% to 71% of patients use one or more forms of CAM at some point in life. However, healthcare professionals are not often aware of CAM used by patients.6-8 Giveon et al.7 pointed out that nearly half of "natural product" users (44.7%) never informed the physicians of their usage, and 29.7 percent of respondents did not think the use of alternative medicine or natural products should be reported to the physicians. Patients´ disclosure of CAM use and reasons for non-disclosure therefore need be fully investigated in order to ensure the patient safety.
Potential adverse effects are a matter of concern among patients using CAM.9,10 These effects may have an impact on patients´ pathology, complications of medical conditions or delay in medical diagnosis. Additionally, apprehensions are particularly reported with patients receiving multiple drug therapy and CAM usage. As for conventional drugs, herbal remedies and dietary supplements (HS) are pharmacologically active and possess some risks of side-effects and interactions with drug therapy.11-13 This could lead to therapeutic failure by reducing systemic absorption or bioavailability of conventional medications, toxicity or an extension of hospital stay, which are all unwanted consequences. The adverse effects of CAM experienced by patients nevertheless remain unclear and merit further investigation.
Many CAM studies5,7,14-16 have been carried out in patients attending GP surgeries or hospital out-patient units, but very few8,17,18 were conducted in hospitalised patients. These few questionnaire surveys mainly focused on the use of CAM and reasons for use. This study was thus intended to investigate the prevalence, disclosure and adverse effects of CAM used by in-patients and to find out any associations between patients´ perceived CAM side-effects and relevant factors. It may enable pharmacists and healthcare professional to gain an insight into patients´ use of CAM, their disclosure and adverse effects that are crucial for patient care and medicines management.
Methods
This cross-sectional study was carried out in hospitalised patients of a district general hospital in the North East of England from November 2008 to November 2009. The study was approved by the National Research Ethics Service Committee (NRES) in the UK and the University´s ethics committee. Other permissions granted included the Caldicott Guardians for personal data protection, NHS Research and Development and audit quality improvement of Northumbria NHS Healthcare Trust.
In this study, the utilisation of "complementary and alternative medicine" (CAM) refers to patients´ use of one or more types of therapies or medicines that are not usually prescribed by general practitioners or pharmacists, and may be bought from health shops. To facilitate the data analysis and interpretation, CAM was divided into two groups: herbal remedies and dietary supplements (HS, e.g. natural products, vitamins and mineral supplements) and other forms of CAM (non-HS, e.g. homeopathy, aromatherapy, massage and acupuncture). Adverse effects here included (1) suspected adverse CAM reactions (or so-called "actual CAM side-effects") reported by in-patients, (2) perceived CAM side-effects and (3) potential interactions between herbal remedies/dietary supplements (HS) and prescribed medicines. For other forms of CAM (non-HS), their interactions with mainstream medicines were not evaluated owing to the incomplete data obtained from hospitalised patients and medical notes; this made it difficult to assess the interactions.
With respect to patients´ reports of actual CAM side-effects, a simple causality assessment that confirmed the association of an adverse event with CAM were performed by using temporal sequence and documented evidence, e.g. reports of side-effects or research papers. Naranjo´s Algorithm20 could not be utilised owing to the incomplete data, such as responses to "dechallenge" or "rechallenge" of CAM. All CAM side-effects were grouped up based on body systems specified in the British National Formulary (BNF)21 and International Classification of Disease (ICD-10).22 Potential interactions between patients´ HS and their allopathic drugs in this study are defined as drug-CAM interactions that are likely to cause patients undesirable effects and can be explained by pharmacokinetics or pharmacodynamics. The adverse interactions were assessed by the researchers using standard information sources, i.e. Stockley´s Herbal Medicine Interaction13 and Medical Pocket Reference: Drug-herb Interactions23, and current evidence.24-26
Patients and eligibility criteria. The study participants were adult in-patients admitted to five specialty wards, i.e. Orthopaedics & Trauma, General Surgery, Elderly Medicine, General Medicine and Gynaecology. These wards were selected so as to provide a broader spectrum of patients than other studies that only emphasised specific patient groups. A few wards, such as Children and Oncology, were excluded, as it was not feasible to obtain patients´ data. Patients were included in the study if they were aged 16 or over and could provide written or oral consent. Exclusion criteria were: a mental health diagnosis, a diagnosis of cancer, a terminal illness, an inability to give informed consent or an inability to communicate with the researchers in English. Medical and nursing staff on the wards could also exclude patients from the study if they felt it was inappropriate in terms of health and safety, as suggested by the ethics committee. In addition to a consent form, a participant information sheet was also provided for patients in order to clarify the purpose of the study, definition and examples of CAM, study activity and risk and benefit of participation, including confidentiality and voluntary withdrawal at any time.
The sample size was estimated based on the prevalence of in-patients´ CAM use and the formula compiled by Eng19, which is the required sample (N)=4(Zcrit)2p(1-p)/d2. Since different studies have different study designs and durations of CAM use, i.e. within 1-2 years or at some point in time, the prevalence rates considerably varied with the studies. The prevalence of 68.0% found in hospitalised patients who ever used CAM8 was chosen on account of its reliable findings. With a 95% confidence interval and its expected width of 10%-20%, the sample size of 84-334 was therefore determined. When taking the incomplete data and patients´ responses into consideration, at least 200 patients were required to detect a prevalence rate in the study.
Study instrument. A semi-structured questionnaire with closed and open-ended questions was specially developed based on the literature evidence. In the questionnaire, the single term "alternative medicine" with a simple definition was used to better communicate with patients. This questionnaire contained three sections. Section 1 embraced patient´s characteristics, whereas Section 2 consisted of the use of CAM and CAM adverse effects. Section 3 contained data extracted from patients´ medical notes, i.e. medical conditions, conventional drug use, records of CAM use, adverse effects, length of hospital stay and outcomes on discharge. The questionnaire was checked for content validity by three experts on complementary therapies, clinical pharmacy and statistics. It was then piloted in 82 patients attending health shops, community pharmacy and hospital, and eventually reviewed by the ethics committee.
Data collection. In-patients who met the eligibility criteria were given unique identification codes, such as R1, R59 or R240. They were initially informed of the consent form and details of the study, and then interviewed face-to-face by the researcher (NB) using the questionnaire for approximately 25-60 minutes. After that, the data found in patients´ medical notes were verified and recorded in Section 3 of the questionnaire. Regarding patient safety, the researchers (NB and WB) would discuss with relevant ward pharmacists any CAM adverse effects reported by patients and resolved the problems accordingly.
Data analysis. All quantitative data from completed questionnaires were entered into PASW Statistics 18 (SPSS-IBM Co., Chicago, IL) and analysed by using descriptive statistics and a Chi-squared test or Fisher´s exact test. A significance level (alpha) was set at 0.05. NVivo 8.0 (QSR International (UK) Ltd., Southport, UK) was employed to manage the qualitative data obtained from open-response questions. Some obscure data from patients´ answers were highlighted for further interpretation. A content analysis with coding schemes based on previous studies and the data was performed by the researcher (NB). For instance, the coding schemes of reasons for disclosure of CAM use included:
"asked by healthcare professionals" (HCP, if asked by healthcare providers, etc.) "willing to answer" (WTA, willing to admit using CAM)
"attitudes of healthcare professionals" (ATT, good attitudes of healthcare professionals towards CAM use)
"approved to use CAM" (APR, approved by healthcare providers to use CAM).
The codes for non-disclosure of CAM application embraced:
"did not consider CAM as medicine" (DCON, did not regard CAM as conventional medicine)
"did not use CAM recently" (DUSE, did not use now so did not inform)
"was not asked specifically about CAM" (WASK, was not asked directly about CAM use)
"might mention if using" (MMEN, might mention if using/taking).
The overall codes and results of the content analysis were rechecked using a small set of the data and eventually confirmed by the research team.
Results
At the outset, 300 in-patients were recruited into the study. Of these, 44 refused to participate, 9 declined to give consent for reviewing medical notes and 7 withdrew from the study during interviews. Thus, a total of 240 patients could be interviewed and their medical notes were accessed. The characteristics of the patients are shown in Table 1. There were slightly more females than males (54.6% vs. 45.4%). Nearly half of them were older patients aged 60 or over (53.3%) with the mean age of 59.3 (SD=17.3 years). They were mostly white British (98.8%) and educated at the college or level (56.7%). Moreover, most patients stayed on three major specialty wards, i.e. Orthopaedics and Trauma (33.3%), General Surgery (30.0%) and General Medicine (29.2%), for the median period of 8 days (interquartile range: 4-14 days).
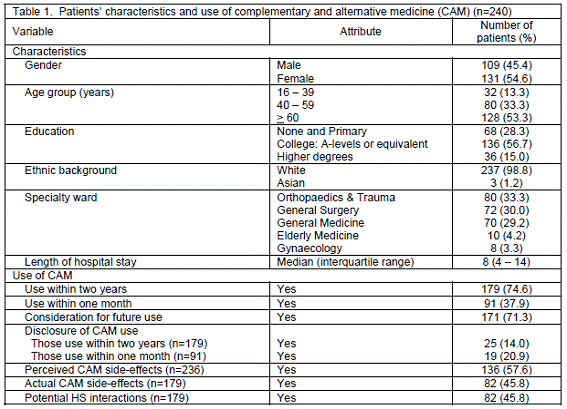
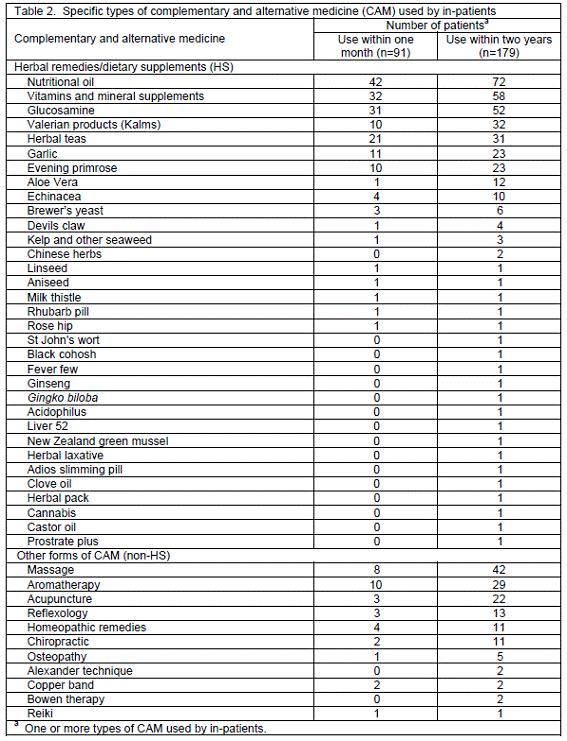
Use of CAM. As demonstrated in Table 1, 179 patients used CAM within two years, but 91 still utilised it within the period of one month prior to the interviews. The prevalence of CAM use within two years and one month was therefore 74.6% and 37.9%, respectively. The specific types of CAM used by the patients are summarised in Table 2. Top 5 HS included nutritional oil (e.g. cod liver oil, omega-3 and seven seas), vitamins and mineral supplements, glucosamine, valerian products and herbal teas, whereas Top 5 non-HS reported were massage, aromatherapy, acupuncture, reflexology and homeopathic remedies. When enquired about the future use of CAM, the majority of patients (71.3%) would consider using it after discharge because of previous experience with CAM effectiveness and less side-effects, professional recommendations, indicated conditions and its availability and accessibility. Some reasons rendered by patients, with their identification codes in the brackets, are as follows:
"...beneficial, harmless, natural, normal, and has less side-effects than my prescription medicines." (R9)
"...will use it if doctors or nurses recommend or prescribe it." (R15)
"...concerned about the safety of convention medicine and once experienced some side-effects, so like to use alternative medicine." (R88)
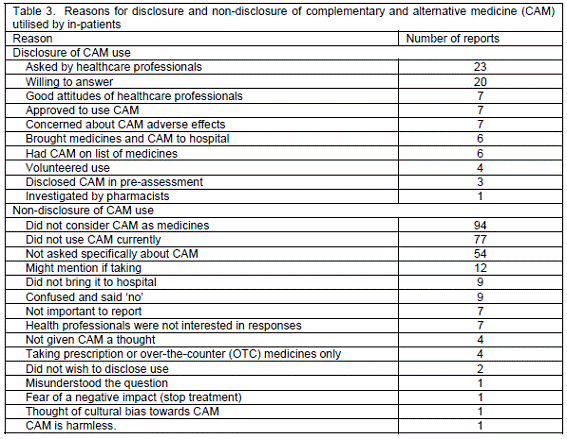
Disclosure of CAM use. For 179 patients who used CAM within two years, 14% informed healthcare professionals of their use of CAM (Table 1). However, 19 of 91 patients (20.9%) who still made use of CAM within one month disclosed their current CAM usage. Their disclosure was also verified through the medical notes. The reasons for disclosure or non-disclosure are presented in Table 3. Patients revealed their CAM use because of their own willingness to answer, good attitudes towards healthcare professionals or the manner of inquiry. On the contrary, they did not notify their CAM applications, since they did not consider CAM as medicines, did not use CAM by the time of inquiry, were not specially asked about CAM or misunderstood the question.
CAM side-effects. In Table 1, slightly more than half (57.6%) perceived CAM might have some side-effects, but the rest had no opinion or were undecided. Patients´ perceptions of CAM side-effects derived from personal experience, the comparison of CAM safety profiles with conventional medicine, discontentment with unqualified CAM practitioners and unrestricted regulations. Views on CAM adverse effects included:
"Lack of knowledge of alternative medicine itself. It might cause some unwanted effects." (R18)
"CAM information is not available like medications and no medical staff involved." (R35)
"It depends on individual sensitivity, personal experience and types." (R107)
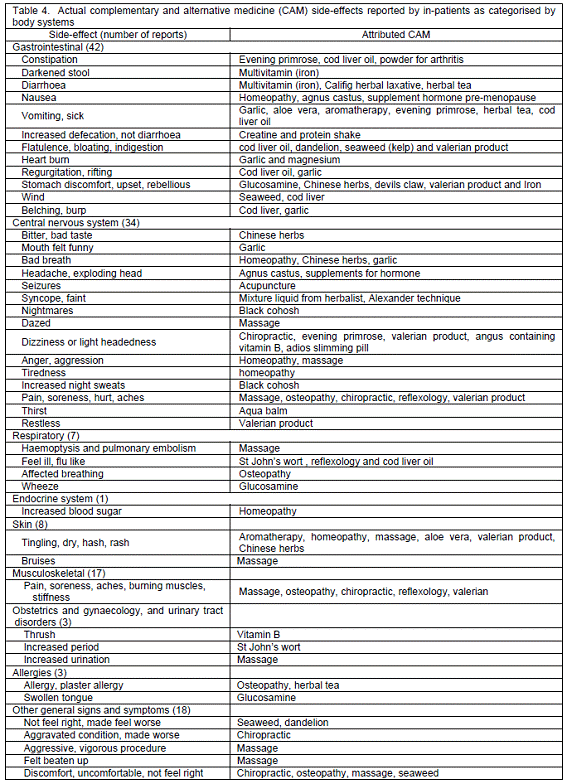
In regard to actual experience with CAM side-effects, 82 patients (45.8%) with 133 adverse events were reported; these included 53 females and 29 males. Their problems tended to resolve or subside on discontinuation (Table 1). All side-effects associated with CAM were regarded as "possible" according to the simple causality assessment, as all patients had received CAM before the adverse effects occurred and there was some documented evidence to support the results. Table 4 summarises actual CAM side-effected notified by the patients as classified by body systems. Most patients encountered gastrointestinal effects, e.g. constipation, darkened stool and diarrhoea, followed by problems in the central nervous system and musculoskeletal system. Examples of attributed CAM with its side-effects are as follows:
Garlic-"...vomiting and mentioned to the doctor who said it might not agree with body, and the reaction subsided when stopped it." (R61)
Massage-"...bringing up blood that was then detected in hospital has clot in lungs." (R154)
Homeopathy-"Reading of blood sugar went high." (R156)
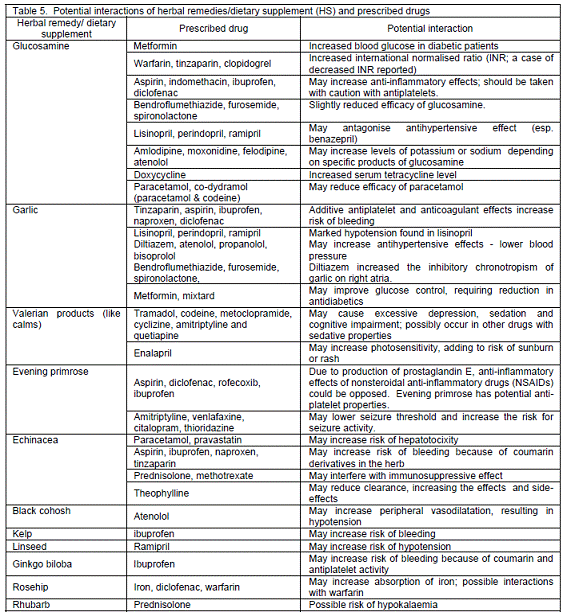
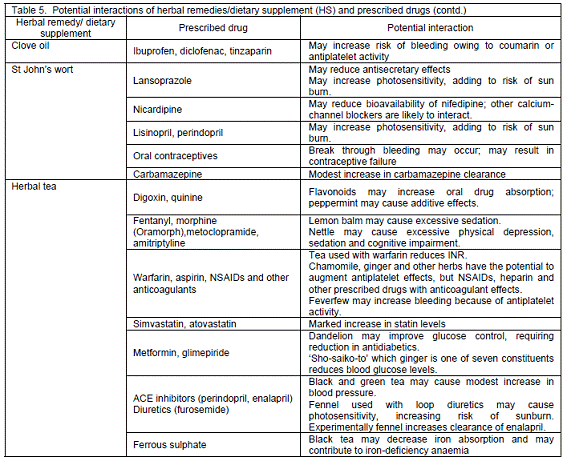
Potential interactions with prescribed drugs. When considering patients´ HS use, potential interactions with prescription drugs could be identified in 82 patients (45.8%). The potential interactions between HS and prescribed medicines are elaborated in Table 5. Most potential interactions were implicated with non-steroidal anti-inflammatory drugs (NSAIDs), anticoagulants, antiplatelets, antihypertensives (e.g. angiotensin-converting enzyme inhibitors) and diuretics. Other significant interactions were concerned with narrow therapeutic index drugs, such as digoxin and theophylline.
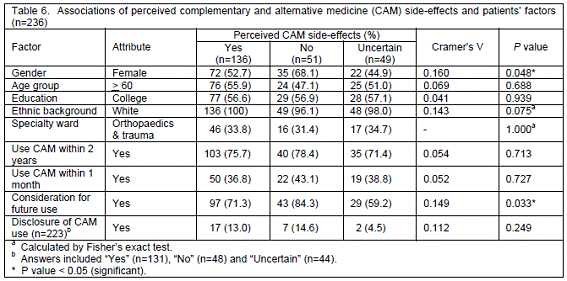
Associations of perceived CAM side-effects and patients´ factors. As shown in Table 6, patients´ perceptions of CAM side-effects were associated with their gender (P=0.048) and consideration for future use of CAM (P=0.033); the strengths of associations as denoted by Cramer´s V values were 16.0% and 14.9%, respectively. Nevertheless, there was no relationship of perceived side-effects with other factors, i.e. the age group, education, ethnic background, specialty wards or use of CAM.
Discussion
The knowledge of CAM used by in-patients in the UK and around the whole has been limited. This study reflected the views of in-patients who were mainly white, older British, which corresponded to the large number of white British in the North East region (92.4%).27 The prevalence of CAM use within two years (74.6%) found in this study was higher than that of other studies in ambulatory and hospitalised patients4-8,14,16, but lower than Shorofi´s study in Australia (90.4%).17 These discrepancies were probably due to the different characteristics of studied population, diverse types of CAM available in the regions and reported duration of CAM use. No prevalence of CAM applications within a month or during admission had been previously reported in the UK, except this study.
With respect to the disclosure of CAM use, there was little evidence of in-patients revealing their applications of CAM during hospital stay in the UK and abroad, and few studies mainly focused on the CAM disclosure to medical doctors and nurses.28,29 Goldstein et al.18 and Cockayne et al.30 indicated that patients´ CAM use history during or prior to admission was rarely recorded in the medical notes. Similar to this study, in-patients´ disclosure rate of 20% or lower was confirmed by other CAM studies.6,8,17 A possible explanation was that patients were not specifically asked about CAM usage, as most healthcare practitioners usually pose the generally question-"Are you taking any other medicines?". They might not regard CAM as either prescription or over-the-counter (OTC) medicines, thus limiting the disclosure of CAM use as ascertained by the study of Adusumilli and his team.6 Moreover, the attitudes of healthcare professionals towards CAM also affected their willingness to unveil their utilisation of CAM. This might also be a reason for under-reporting of CAM adverse effects.
Slightly more than half of the patients (57.6%) were aware of CAM side-effects and their perceptions were associated with gender and consideration for future use. Compared with male patients who were mostly not sure about CAM side-effects, females were likely to perceive the adverse effects, albeit less harmful and use more CAM. The frequent use of CAM as well as health services in general by female patients was confirmed by Murray and Shepherd.31 Additionally, patients who thought about using CAM in the future tended to express their views on CAM side-effects, which were less than conventional drugs. Interestingly, the association between the previous or present use of CAM and perceived side-effects was not found in this study, possibly because most patients were not certain about CAM adverse effects, i.e. whether it could really cause a problem. This could be another possible explanation for under-reporting of adverse drug or CAM effects.32
Although some patients found it difficult to describe CAM side-effects, nearly half of patients (45.8%) who used CAM within two years could report 133 adverse events. These side-effects were largely mild to moderate with only few serious or life-threatening events, e.g. haemoptysis by patient with pulmonary embolism resulted from massage and syncope as a result of Alexander technique. This result differed from previous studies6,8 that only documented theoretically potential adverse effects based on patients´ use of CAM. A recent study however published side-effects observed by ambulatory hypertensive patients.16 It should be noted that the adverse CAM reactions (or "actual CAM side-effects) experienced by patients could not be classified as Type A or B according to the Rawlins-Thompson classification33 due to the complexity of the events. With limited information, the CAM practices or medicines were however considered as "possible" causes of adverse effects based on the simple causality assessment.
The incidence of potential interactions between HS and conventional drugs is low15,18 and remains unreported in hospitalised patients. An attempt was made in this study to assess the theoretical potential interactions, but there might be risk of exaggerating or underestimating the interactions due to researchers´ background or unavailable evidence. Whether drug-CAM interactions are clinically significant, it should be prudent when managing patients´ concomitant use of CAM and conventional drugs.13 In this study, grape fruit juice was not clearly specified by patients, as they might occasionally use it alone or as a constituent in herbal teas. Its interactions with conventional drugs are of paramount important owing to enzyme inhibitory effects.34
Limitations of the study. The main limitations included patients´ recall bias and interpretations of open-response questions. Some patients were unable to memorise the use of CAM within two years or elaborate the specific type of CAM. The bias therefore impacted on the documentation of CAM use. Since patients were allowed to express their views freely on all queries, some responded with the answers "yes" together with explanations of "no", or vice versa. These ambiguous responses made it very challenging to perform the content analysis. As there were many activities on the wards, i.e. morning and afternoon ward rounds, meal times, family visiting and patient rest periods, an appropriate time slot for interviews was usually between 11 am and 12 noon during weekdays. It thus took very long time to finish up all interviews. Additionally, the findings might not be fully applicable to the non-white population, or ethnic minorities, who normally utilise CAM, for the majority of patients in this study were white British that were consistent with the population demographics in the North East region.
Conclusions
This study could investigate the prevalence, disclosure and adverse effects of CAM use in hospitalised patients and the associations of perceived side-effects with patients´ gender and consideration for future use. Healthcare professionals should encourage patients to unveil their CAM use and any suspected adverse effects by self-reporting. The uncovered data must be also recorded in medical notes or via the Yellow Card Scheme35, which is a spontaneous reporting system for collecting information on suspected adverse drug reactions (ADRs) to medicines in the UK. Furthermore, potential interactions between CAM and conventional drugs or underlying diseases should be assessed on a regular basis. As part of a pharmacovigilance system in hospital, pharmacists can have a role in monitoring patients´ CAM use in order to ensure patient safety and effective medicines management. It is essential that regulatory standards to balance the CAM safety, availability and accessibility to the public and patients are taken into account by health policy makers. Further studies are needed to evaluate specific CAM usage and related adverse events in specific groups of patients to ascertain the CAM effectiveness and safety.
Acknowledgements
We would like to thank Northumbria Healthcare NHS Trust, North Tyneside General Hospital and the University of Sunderland for approving this research. We were also grateful to the patients who participated in this study and to Dr John Lough and Dr Alan Worsley for their thoughtful advice and support.
Conflict of interest
We authors declare that we have no conflict of interest to disclose.
References
1. World Health Organization. General guidelines for methodologies on research and evaluation of traditional medicines (WHO/EDM/TRM/2000.1). Geneva: WHO; 2000. [ Links ]
2. Kayne SB. Complementary therapies for pharmacists. London: Pharmaceutical Press; 2002. [ Links ]
3. National Center for Complementary and Alternative Medicine. What is complementary and alternative medicine? Bethesda, MD: NCCAM; 2010. http://nccam.nih.gov/health/whatiscam (accessed on July 6, 2011). [ Links ]
4. Ernst E. The usage of complementary therapies by dermatological patients: a systematic review. Br J Dermatol. 2000;142(5):857-861. [ Links ]
5. Featherstone C, Godden D, Gault C, Emslie M, Took-Zozaya M. Prevalence study of concurrent use of complementary and alternative medicine in patients attending primary care services in Scotland. Am J Public Health. 2003;93(7):1080-1082. [ Links ]
6. Adusumilli PS, Ben-Porat L, Pereira M, Roesler D, Leitman IM. The prevalence and predictors of herbal medicine use in surgical patients. J Am Coll Surg. 2004;198(4):583-590. [ Links ]
7. Giveon SM, Liberman N, Klang S, Kahan E. Are people who use "natural drugs" aware of their potentially harmful side-effects and reporting to family physician? Patient Educ Couns. 2004;53(1):5-11. [ Links ]
8. Shakeel M, Bruce J, Jehan S, McAdam TK, Bruce DM. Use of complementary and alternative medicine by patients admitted to a surgical unit in Scotland. Ann R Coll Surg Engl. 2008;90(6):571-576. [ Links ]
9. Cosyns J, Jadoul M, Squifflet J, Wese F, van Ypersele de Strihou C. Urothelial lesions in Chinese-herb nephropathy. Am J Kidney Dis. 1999;33(6):1011-1017. [ Links ]
10. Mikhail A, Reidy JF, Taylor PR, Scoble JE. Renal artery embolization after back massage in a patient with aortic occlusion. Nephrol Dial Transplant. 1997;12(4):797-798. [ Links ]
11. Ernst E. Adulteration of Chinese herbal medicines with synthetic drugs: a systematic review. J Intern Med. 2002;252(2):107-113. [ Links ]
12. Gilani AH, Atta-ur-Rahman. Trends in ethnopharmacology. J Ethnopharmacol. 2005;100(1-2):43-49. [ Links ]
13. Williamson EM, Driver S, Baxter K. Stockley's herbal medicines interactions. London: Pharmaceutical Press; 2009. [ Links ]
14. Lewith GT, Broomfield J, Prescott P. Complementary cancer care in Southampton: a survey of staff and patients. Complement Ther Med. 2002;10(2):100-106. [ Links ]
15. Peng CC, Glassman PA, Trilli LE, Hayes-Hunter J, Good CB. Incidence and severity of potential drug-dietary supplement interactions in primary care patients: an exploratory study of 2 outpatient practices. Arch Intern Med. 2004;164(6):630-636. [ Links ]
16. Olisa NS, Oyelola FT. Evaluation of use of herbal medicines among ambulatory hypertensive patients attending a secondary health care facility in Nigeria. Int J Pharm Pract. 2009;17(2):101-105. [ Links ]
17. Shorofi SA, Arbon P. Complementary and alternative medicine (CAM) among hospitalised patients: an Australian study. Complement Ther Clin Pract. 2010;16(2):86-91. [ Links ]
18. Goldstein LH, Elias M, Ron-Avraham G, Biniaurishvili BZ, Madjar M, Kamargash I, Braunstein R, Berkovitch M, Golik A. Consumption of herbal remedies and dietary supplements amongst patients hospitalized in medical wards. Br J Clin Pharmacol. 2007;64(3):373-380. [ Links ]
19. Eng J. Sample size estimation: how many individuals should be studied? Radiol. 2003;227(2):309-313. [ Links ]
20. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245. [ Links ]
21. The British Medical Association and the Royal Pharmaceutical Society. British national formulary no. 61. London: RPS; 2011. http://www.bnf.org (accessed on July 6, 2011). [ Links ]
22. World Health Organization. International classification of diseases v.10 (ICD-10). Geneva: WHO; 2011. http://www.who.int/classifications/icd/en (accessed on July 6, 2011). [ Links ]
23. Springhouse, editor. Medical pocket reference: drug-herb Interactions. New York: Lippincott Williams & Wilkins; 2002. [ Links ]
24. Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs. 2001;61(15):2163-2175. [ Links ]
25. Peng CC, Glassman PA, Trilli LE, Hates-Hunter J, Good CB. Incidence and severity of potential drug-dietary supplement interactions in primary care patients: an exploratory study of two outpatient practices. Arch Intern Med. 2004;164(6):630-636. [ Links ]
26. Taylor DM, Walsham N, Tayleo SE, Wong L. Potential interactions between prescription drugs and complementary and alternative medicines among patients in the Emergency Department. Pharmacotherapy. 2006;26(5):634-640. [ Links ]
27. Office for National Statistics. Statistical bulletin: population estimates by ethnic group 2002-2009. London: ONS; 2011. [ Links ]
28. Barraco D, Valencia G, Riba AL, Nareddy S, Draus CBSN, Schwartz SM. Complementary and alternative medicine (CAM) use patterns and disclosure to physicians in acute coronary syndromes patients. Complement Ther Med. 2005;13(1):34-40. [ Links ]
29. Liu C, Yang Y, Gange SJ, Weber K, Sharp GB, Wilson TE, Levine A, Robison E, Goparaju L, Gandhi M, Merenstein D. Disclosure of complementary and alternative medicine use to health care providers among HIV-Infected women. AIDS Patient Care STDS. 2009;23(11):965-971. [ Links ]
30. Cockayne NL, Duguid M, Shenfield GM. Health professionals rarely record history of complementary and alternative medicines. Br J Clin Pharmacol. 2005;59(2):254-258. [ Links ]
31. Murray J, Shepherd S. Alternative or additional medicine? An exploratory study in general practice. Soc Sci Med. 1993;37(8):983-988. [ Links ]
32. Barnes J. Pharmacovigilance of herbal medicines: A UK perspective. Drug Saf. 2003;26(12):829-851. [ Links ]
33. Davies DM, Ferner RE, de Glanville H. Davies´s textbook of adverse drug reactions. 5th ed. London: Chapman & Hall Medical; 1998. [ Links ]
34. Bailey DG, Malcolm J, Arnold O, David-Spence J. Grapefruit juice-drug interactions. Br J Clin Pharmacol. 1998;46(2):101-110. [ Links ]
35. Medicines and Healthcare products Regulatory Agency (MHRA). Yellow Card Scheme. London: MHRA; 2011. http://www.mhra.gov.uk/Safetyinformation/Howwemonitorthesafetyofproducts/Medicines/TheYellowCardScheme/index.htm (accessed on July 6, 2011). [ Links ]
Received (first version): 21.12.11
Accepted: 19.07.12














