Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Pharmacy Practice (Granada)
versión On-line ISSN 1886-3655versión impresa ISSN 1885-642X
Pharmacy Pract (Granada) vol.10 no.4 Redondela oct./dic. 2012
ORIGINAL RESEARCH
An analysis of the warning letters issued by the FDA to pharmaceutical manufacturers regarding misleading health outcomes claims
Análisis de las cartas de advertencia enviadas por la FDA a industrias farmacéuticas sobre propagandas engañosas de resultados en salud
Satabdi Chatterjee1, Harshali K. Patel2 and Sujit S. Sansgiry3.
1MS. Department of Clinical Sciences and Administration, College of Pharmacy, University of Houston. Houston (United States)
2MS. Department of Clinical Sciences and Administration, College of Pharmacy, University of Houston. Houston (United States)
3PhD. Associate Professor. Department of Clinical Sciences and Administration, College of Pharmacy, University of Houston. Houston (United States)
SUMMARY
Objective: To evaluate the number and type of warning letters issued by the US Food and Drug Administration (FDA) to pharmaceutical manufacturers for promotional violations.
Methods: Two reviewers downloaded, printed and independently evaluated warning letters issued by the FDA to pharmaceutical manufacturers from years 2003-2008. Misleading claims were broadly classified as clinical, Quality-of-Life (QoL), and economic claims. Clinical claims included claims regarding unsubstantiated efficacy, safety and tolerability, superiority, broadening of indication and/or omission of risk information. QoL claims included unsubstantiated quality of life and/or health-related quality of life claims. Economic claims included any form of claim made on behalf of the pharmaceutical companies related to cost superiority of or cost savings from the drug compared to other drugs in the market.
Results: In the 6-year study period, 65 warning letters were issued by FDA, which contained 144 clinical, three QoL, and one economic claim. On an average, 11 warning letters were issued per year. Omission of risk information was the most frequently violated claim (30.6%) followed by unsubstantiated efficacy claims (18.6%). Warning letters were primarily directed to manufacturers of cardiovascular (14.6%), anti-microbial (14.6%), and CNS (12.5%) drugs. Majority of the claims referenced in warning letters contained promotional materials directed to physicians (57%).
Conclusion: The study found that misleading clinical outcome claims formed the majority of the promotional violations, and majority of the claims were directed to physicians. Since inadequate promotion of medications may lead to irrational prescribing, the study emphasizes the importance of disseminating reliable, credible, and scientific information to patients, and more importantly, physicians to protect public health.
Key words: Advertising as Topic. Marketing. Drug Industry. United States Food and Drug Administration. United States.
RESUMEN
Objetivo: Evaluar el número y el tipo de cartas de advertencia enviadas por la US Food and Drug Admisnitration (FDA) a industrias farmacéuticas por violaciones promocionales.
Métodos: Dos revisores descargaron, imprimieron y evaluaron independientemente las cartas de advertencia enviadas por la FDA a industrias farmacéuticas entre os años 2003-2008. Las propagandas engañosas se clasificaron como propagandas clínicas, de calidad de vida (QoL) y económicas. Las propagandas clínicas incluían propagandas de eficacia, seguridad y tolerabilidad, superioridad, amplitud de indicación no sustanciadas, y/u omisión de información de riesgos. Las propagandas de QoL incluían propagandas de calidad de vida o de calidad de vida relacionada con la salud no sustanciadas. Las propagandas económicas incluían cualquier forma de propaganda de las compañías farmacéuticas relacionada con superioridad de costes o ahorro de costes del medicamento comparado con otros en el mercado.
Resultados: En los 6 años de estudio, la FDA envió 65 cartas de advertencia que contenían 144 propagandas clínicas, 3 de QoL, y una económica. De media, se enviaron 11 cartas de advertencia por año. La violación más frecuentemente encontrada fue la omisión de la información de riesgo (30,6%), seguida de propagandas de eficacia insustanciadas (18,6%). Las cartas de advertencia fueron dirigidas principalmente a medicamentos cardiovasculares (14,6%), antimicrobianos (14,6%) y del SNC (12,5%). La mayoría de las propagandas referidas en las cartas contenía materiales promocionales dirigidos a los médicos (57%).
Conclusión: El estudio encontró que las propagandas engañosas de resultados clínicos consituian la mayoría de las violaciones promocionales, y la mayoría de estas propagandas estaban dirigidas a médicos. Como la promoción inadecuada puede llevar a una prescripción irracional, este estudio enfatiza la importancia de diseminar información fiable, creíble y científica a los pacientes, y más importante a los médicos para así proteger la salud pública,.
Palabras clave: Publicidad como Asunto. Marketing. Industria Farmacéutica. United States Food and Drug Administration. Estados Unidos.
Introduction
Pharmaceutical manufacturers communicate the drug's intended efficacy and benefits to physicians and patients through promotional claims for the particular product.1-3 The various promotional materials include print advertisements, broadcast advertisements, and visual aids which are commonly used by sales representatives around the world as part of their product promotion strategy.1-3 These claims should ideally be based on scientific evidence since they form the basis of medication prescribing by physicians, and utilization by patients. All promotional claims that are made by or on behalf of global drug manufacturers in the United States (US) are subject to regulation by the US Food and Drug Administration (FDA).4-6 The Division of Drug, Marketing, Advertising and Communications (DDMAC) is the unit within the FDA's Center for Drug Evaluation and Research (CDER), which is concerned with the regulation of prescription drug promotion and oversees all the promotional activities.7-8
In contrast to countries that rely mainly on industry self-regulation, the US-FDA directly monitors promotion of prescription drugs.9 The FDA can employ a number of enforcement tools when they perceive a promotional claim violation, depending on the degree of severity of the claim violated, the previous track record of the individual company, and its past confrontation with FDA.10 Warning letters constitute one of the most frequently pursued advisory actions by the FDA. According to the definition given by Department of Scientific Investigation (DSI), a warning letter "is an informal advisory, to a firm or clinical investigator, communicating the Agency's position on a matter but does not commit the FDA to take enforcement action".10 The company receiving a warning letter is required to reply within 15 days, immediately ceasing the circulation of the material or action in violation or issue a -Dear Doctor" letter or take any corrective action as required.10 Past research suggests that lack of standardized FDA guidelines for reviewing warning letters may have led to use of more informal criteria while overseeing drug marketing and promotional activities.4
The US Department of Health and Human Services (DHHS) and the Pharmaceutical Research and Manufacturers of America (PhRMA) implemented two policies in 2002 and 2005 respectively, pertaining to the oversight of warning letters and promotion of claims.11 In the light of informal criteria adopted by FDA officials for reviewing regulatory letters, the DHHS policy, implemented in January 2002, required the Office of Chief Counsel to review these letters before they were issued by the DDMAC.11 In 2005, the PhRMA issued guidance effective January 2006, which required pharmaceutical companies to submit all new Direct to Consumer (DTC) television drug advertisements to the FDA prior to broadcasting them. Therefore, the objective of the current study was to evaluate the number and type of warning letters issued by the FDA. Specifically, the aim was to identify the primary focus of promotional claim violations in warning letters, whether it was clinical, quality of life, or economic. Further, the most common drug classes associated with the warning letters and the intended audience for the claims was also assessed.
Methods
This study employed a content analysis approach to review warning letters issued by the FDA during 2003 through 2008 where replicable and valid inferences from the text were made.12 First, investigators downloaded and printed all warning letters sent to pharmaceutical companies from the website http://www.fda.gov/cder/warn. A data abstraction form collected information on drug name, company name, therapeutic class, number of claims, type of promotional material, claim category, and target audience for these promotional claims. Claims were classified based on the Economic, Clinical, and Humanistic Outcomes model, namely misleading economic, clinical, and QoL outcome claims.13
The misleading clinical outcome claims category was sub-classified into 5 groups based on DDMAC's focus of activities in recent years: 1) Overstatement of efficacy or unsubstantiated efficacy/effectiveness claims, 2) Comparison of clinical effectiveness/superiority claims, 3) Unsubstantiated safety and tolerability claims, 4) Broadening of indication claims, and 5) Omission or minimization of risk information.14 Misleading QoL outcome claims were divided into: 1) Unsubstantiated QoL claims and 2) Misleading claims of physical functioning/Health related quality-of-life (HRQoL). Misleading economic claims included any form of claim made on behalf of the pharmaceutical companies related to cost superiority of or cost savings from the drug compared to other drugs in the market. Claims that did not fall within the above classification were categorized as "other claims" and it included issues such as failure to submit the material at the time of initial distribution, omission of material facts, omission of indication of use, and promotion of an investigational new drug. Depending on the frequency of occurrence in the warning letters and based on the prevalence of diseases in US, therapeutic classes were broadly classified into anti-cancer drugs, HIV drugs, cardiovascular (CVS) agents; central nervous system (CNS) agents, upper respiratory tract infection (URTI) agents, analgesics, anti-microbial agents, synthetic hormones, and others.15,16
Each warning letter was reviewed by two individuals and entered into a database in Microsoft Excel 2007. A third reviewer arbitrated any form of disagreement. Descriptive analysis was conducted using SAS 9.1, to determine the total number of claims in each letter, the total number of promotional claims per year throughout the study period, the number of letters directed to physicians or patients, and the therapeutic categories of the drugs which received the warning letters for misleading claims.
The study considered only those warning letters sent to pharmaceutical companies for promotional materials disseminated in the US and which made false/misleading claims, during the years 2003 through 2008. Consequently, the authors excluded all other notices and warning letters not addressed to a pharmaceutical company during the study period.
Results
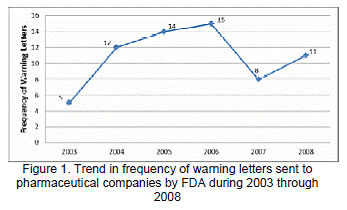
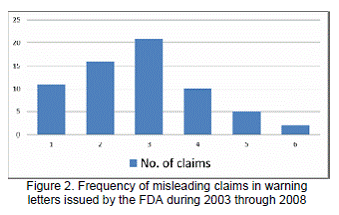
In this study, the researchers reviewed 65 warning letters that contained 144 (78.7%) misleading clinical, three (1.6%) QoL, and one (0.5%) economic claim violations. Figure 1 shows the trend in frequency of warning letters issued by the FDA each year. On an average, 11 warning letters were issued each year over the study period. Although, there was an increase in the number of warning letters from 2003-2006, this trend did not continue in 2007. There was decrease of 46.6% in the number of warning letters from 2006 to 2007, which again increased by 37.5% in 2008.The number of claims in each warning letter varied from 1 to as many as 6 claims. Figure 2 provides the distribution of claims per letter. There were more letters with multiple claims (83%). Over 55% had 2 to 3 claims per letter.
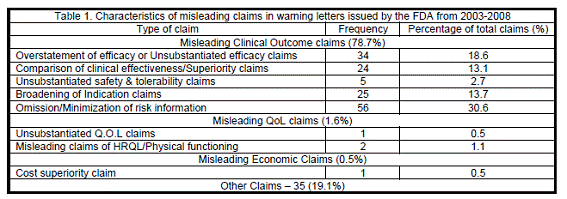
As shown in Table 1, for clinical outcome claims, the highest frequency was seen for omission or minimization of risk information (30.6% of the total number of violations), followed by overstatement of efficacy or unsubstantiated efficacy claims (18.6% of the total claims).
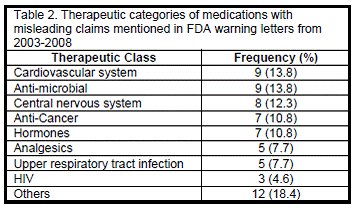
Table 2 shows the therapeutic categories of the drugs, which received warning letters in the study period. Majority of these letters were issued to manufacturers of cardiovascular (13.8%), antimicrobial (13.8%), and CNS agents (12.3%).
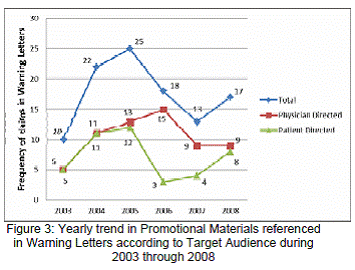
Figure 3 shows the frequency and percentage of promotional materials referenced in the warning letters by the type of audience targeted (physicians or patients). A total of 105 materials were referenced in the warning letters. Of which, 57% (62) had promotional materials directed to physicians, using journal advertisements, professional file cards, and "Dear Healthcare Provider"/ "Dear Doctor" materials. Nearly 43% (43) of the references in the warning letters were directed to patients using website, TV and radio advertisements, and patient brochures.
Discussion
An average of 11 warning letters per year was observed in the study, which was higher than the average of 2 reported in a previous study that evaluated warning letters from 1997-2002.17 Our study found highest frequency of clinical outcome related claims, with omission of risk information being the most frequently violated claim, followed by overstatement of efficacy/unsubstantiated efficacy claims, and comparison of clinical effectiveness claims.
The high frequency of various misleading clinical outcome claims in our study may be attributed to increased FDA surveillance in protecting public health. As indicated by past research2,3, pharmaceutical manufacturers make such claims in order to gain an advantage over competing product(s) in the market. Research also suggests that, consumers are more receptive to such persuasive claims compared to information-based product advertising.18 Hence, advertisements focusing on overstatement of drug efficacy/effectiveness, broadened indication, and minimal risk garner more consumer attention, and reduces the consumer's ability to gather the actual information, which may be detrimental to public health. Based on available evidence and past research, it is important that pharmaceutical companies recognize the importance of communicating scientific information adequately to the target audience to protect public health. Further, there have been no formal FDA guidelines available till date regarding what constitutes an appropriate non-violative claim directed at healthcare professionals and patients.1 This may be partly responsible for the high frequency of misleading clinical claims in our study. The absence of FDA guidelines on the proper nature of a claim could possibly lead to confusion and uncertainty among pharmaceutical companies, and hence the high number of claims. Development of formal FDA guidelines on the proper nature of a promotional claim can play an important role in ensuring patient safety, and protecting public health.
The current study found very few economic or QoL claims. This can be attributed to the enactment of FDA Modernization Act (FDAMA, Section 114) in 1997 which permitted pharmaceutical manufacturers to disseminate Health Care Economic Information (HCEI) if it is supported by -competent and reliable scientific evidence' rather than the established standard of substantial evidence.1-3,19 However, it should be noted in this context that, the FDAMA provided guidelines to disseminate HCEI to only managed care formularies and similar entities.
The higher proportion of warning letters for misleading claims included physician directed promotional materials (Figure 3). This could indicate that, the promotional materials directed at physicians have been a major focus of DDMAC's surveillance activities in recent years. Past research suggests that, although DTC advertising has seen a considerable rise in the past decade, physician directed advertising still remains a powerful source of drug advertising which accounts for majority of the pharmaceutical expenditures.20 The consequences of physician directed advertising generates serious concern. Previous studies have demonstrated that promotions by pharmaceutical representatives, poor quality of drug information in journals, broadcast and other media may potentially impact physician practices.21,22
The finding that relatively high numbers of warning letters were issued in therapeutic categories like cardiovascular, anti-cancer, anti-microbial and CNS agents implies that a substantial section of the US population could be exposed to misleading drug information from various sources. Overall, the current study emphasizes the importance of disseminating reliable, credible, and scientific information, to the target audience, particularly to the prescribers as an effective means to promote rational prescribing, and thereby protect public health. Future studies can focus on the education of prescribers regarding drug promotion and also development of effective guidelines to help the physicians and eventually, the consumers in seeking appropriate drug-related information.
Although the study revealed important findings, several limitations must be acknowledged. First, there was no information available on the total number of promotional materials or warning letters actually submitted to the DDMAC. This was due to lack of data regarding the interaction between DDMAC and pharmaceutical companies which did not result in a warning letter. There were several letters in which a particular claim violated could be placed in more than one category. In such cases, claims were classified based on subjective judgment, based on general consensus among reviewers. It was also stated on the FDA website that some of the warning letters were edited from list in order to remove confidential information. This may lead to underestimating the number of claims or warning letters in general. Finally, our study findings can only be generalized to the particular study period (2003-2008), and not to any prior or subsequent time periods.
Conclusions
The current study found a relatively higher number of warning letters issued by the FDA from 2003 to 2008 compared to previous years (1997-2002), which can be attributed to FDA's surveillance activities in protecting public health. Majority of the misleading claims were clinical in nature and contained promotional materials directed to physicians. Since inadequate promotion of medications may lead to irrational prescribing, the current study emphasizes the importance of disseminating reliable, credible, and scientific information, to the target audience, particularly to the prescribers as an effective means to promote rational prescribing, and thereby protect public health.
Conflict of interest
The authors do not have conflicts of interest related to this research or manuscript.
Funding source: None.
References
1. Stewart KA, Neumann PJ. FDA Actions against Misleading or Unsubstantiated Economic and Quality-of-Life Promotional Claims: An Analysis of Warning Letters and Notices of Violation. Value Health. 2002;5(5):390-395. [ Links ]
2. Kamal KM, Deselle SD, Rane P, Parekh R, Zacker C. Content Analysis of FDA warning letters to Manufacturers of Pharmaceuticals and Therapeutic Biologicals for Promotional Violations. Drug Inf J. 2009;43:385-393. [ Links ]
3. Benson EB, Alfors SN. Prescription drug advertising and promotion: learnings from recent Food and Drug Administration warning letters. Drug Inf J. 2007;41:281-289. [ Links ]
4. Berry IR, Martin RP. The Pharmaceutical Regulatory Process. Second Edition. Informa Health Care. 2008; 371-386. [ Links ]
5. Berry IR. The Pharmaceutical Regulatory Process. Taylor & Francis e-Library.2005; 609-651. [ Links ]
6. Borchers AT, Hagie F, Keen CL, Gershwin ME. The History and Contemporary Challenges of the US Food and Drug Administration. Clin Ther. 2007;29(1):1-16. [ Links ]
7. Center for Drug Evaluation and Research, Division of Drug, Marketing, Advertising and Communications. Description .Silver Spring (MD): US FDA. http://fda.gov/cder/ddmac/ (Accessed April 2009) [ Links ]
8. Center for Drug Evaluation and Research, Division of Drug Marketing, Advertising and Communications. CDER Handbook: Warning Letter. Rockville (MD): US FDA. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/DrugMarketingAdvertisingandCommunications/ucm081617.htm (Accessed November 2009) [ Links ]
9. Mintzes B. Disease Mongering in Drug Promotion: Do Governments Have a Regulatory Role? PLoS Med. 2006;3(4):e198. [ Links ]
10. Center for Drug Evaluation and Research, Division of Drug, Marketing, Advertising and Communications. Description. Silver Spring, (MD): US FDA. http://www.fda.gov/AboutFDA/CentersOffices/CDER/ucm090279.htm (Accessed November 2009) [ Links ]
11. United States Government Accountability Office. GAO-08-758T. Prescription Drugs: Trends in Oversight of Direct-to-Consumer Advertising. May 8, 2008. [ Links ]
12. Klaus Krippendorf. Content analysis: An Introduction to its methodology. Second edition. Thousand Oaks, CA: Sage Publications. 2008. [ Links ]
13. Gunter MJ. The role of the ECHO model in outcomes research and clinical practice improvement. Am J Manag Care. 1999;5(4 Suppl):S217-24. [ Links ]
14. Recent trends in DDMAC enforcement activity. (Aug 28, 2006) Food and Drug e-Alert. http://www.cov.com/files/Publication/0dd2bef8-c1d1-44a5-a7aa-9871458b0088/Presentation/PublicationAttachment/2b5fe504-baa4-4553-9db8-7f9505ed368a/665.pdf (Accessed October 2009) [ Links ]
15. The Best-Selling drugs In America. Pharmaceuticals. (Feb 26, 2006) http://www.forbes.com/2006/02/27/pfizer-merck-genentech-cx_mh_0224topsellingdrugs.html (Accessed April 2009) [ Links ]
16. Partnership to Fight Chronic Disease. http://www.fightchronicdisease.org/ (Accessed April 2009) [ Links ]
17. Trends in DDMAC Enforcement Activity During the Bush Administration. Food and Drug e-Alert; March 30, 2009. http://www.cov.com/files/Publication/6532be42-767e-4c2c-acc0-0c350eed5d8e/Presentation/PublicationAttachment/04b38cf7-655e-45d8-a98d-1cd0f595f212/Trends in DDMAC Enforcement Activity During the Bush Administration.pdf (Accessed November 2009) [ Links ]
18. Holmes ER, Desselle SP. Evaluating the balance of informative and persuasive content within product-specific print DTC ads. Drug Inf J. 2004;38:83-98. [ Links ]
19. Jackson J. FDAMA 1997 Section 114: Another Look. Value Health.2009;12(2):1-2. [ Links ]
20. Gellad ZF, Lyles KW. Direct-to-consumer advertising of pharmaceuticals. Am J Med. 2007;120(6):475-480. [ Links ]
21. Cardarelli R, Licciardone JC, Taylor LG. A cross-sectional evidence-based review of pharmaceutical promotional marketing brochures and their underlying studies: is what they tell us important and true? BMC Fam Pract. 2006;7:13. [ Links ]
22. Drug Promotion what we know, what we have yet to learn. (2004) http://apps.who.int/medicinedocs/pdf/s8109e/s8109e.pdf (Accessed July 2011) [ Links ]
Received (first version): 01.04.12
Accepted: 07.11.12