My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Pharmacy Practice (Granada)
On-line version ISSN 1886-3655Print version ISSN 1885-642X
Pharmacy Pract (Granada) vol.13 n.1 Redondela Jan./Mar. 2015
ORIGINAL RESEARCH
Evaluation of the occurrence and type of antiretroviral and opportunistic infection medication errors within the inpatient setting
Evaluación de la aparición y tipo de errores de medicación de antiretrovirales y medicamentos para infección oportunistas en el entorno hospitalario
Thomas D. Chiampas1, Hajwa Kim2, Melissa Badowski3
1PharmD, BCPS, AAHIVP. Clinical Assistant Professor. College of Pharmacy, University of Illinois at Chicago. Chicago (United States). tchiamp2@uic.edu
2MS. Center for Clinical and Translational Science, University of Illinois at Chicago. Chicago (United States). hkim288@uic.edu
3PharmD, BCPS, AAHIVP. Clinical Assistant Professor. College of Pharmacy, University of Illinois at Chicago. Chicago (United States). badowski@uic.edu
ABSTRACT
Background: Previous data reports inpatient antiretroviral (ARV) and opportunistic infection (OI) medication errors in as many as 86% of patients, with averages ranging from 1.16-2.7 errors/patient.
Objective: To determine the occurrence and type of inpatient ARV and OI medication errors at our institution.
Methods: A retrospective, observational, electronic medical chart review of patients with HIV/AIDS admitted between February 15, 2011- May 22, 2012 was conducted to assess the occurrence and type of ARV and OI medication errors. Secondary outcomes included assessing each medication with an error and evaluating its potential for a medication error, calculating a medication error rate per patient, evaluating whether a non-formulary (NF) medication impacted the error potential, determining whether a clinical pharmacist on service decreased the medication error rate, and assessing whether patients who experienced an error were more likely to have a longer length of stay (LOS). Analysis included descriptive statistics, averages, and Spearmen rank correlation.
Results: There were 344 patients included in this analysis, 132 (38%) experienced 190 medication errors (1.44 errors/patient). An omitted order was the most frequent ARV error and accounted for 30% (n=57) of total errors. There were 166 patients requiring OI medications, 37 patients experienced 39 medication errors. Omitting OI prophylaxis accounted for 31 errors. Only 45 of 190 (24%) errors were corrected prior to discharge. Being prescribed at least 1 NF medication was correlated with increased errors (n=193 patients "on NF medication", p<0.025, r=0.12). Coverage of a service by a clinical pharmacist did not affect the number of errors. Patients experiencing an error had a longer LOS (p=0.02).
Conclusions: Errors relating to ARV and OI medications are frequent in HIV-infected inpatients. More errors occurred in patients receiving NF medications. Suggested interventions include formulary revision, education, and training. Dedicated HIV clinicians with adequate training and credentialing may improve the management of this specialized disease state.
Key words: Medication Errors; Anti-Retroviral Agents; HIV Infections; Inpatients; United States.
RESUMEN
Antecedentes: Datos existentes confirman que los errores de medicación en pacientes hospitalizados con antiretrovirales (ARV) e infecciones oportunistas (IO) aparecen en tantos como el 86% de los pacientes, con medias que oscilan entre 1,16 y 2,7 erroers por paciente.
Objetivo: Determinar la aparición y el tipo de errores de medicación ARV y de IO en nuestra institución.
Métodos: Se realizó una revisión retrospectiva y observacional de las historias clínicas electrónicas de los pacientes con VIH/SIDA ingresados entre el 15 de febrero de 2011 y el 22 de mayo de 2012, para evaluar la aparición y el tipo de errores de medicación ARV y de IO. Los resultados secundarios incluían evaluar las medicaciones con error y evaluar su posibilidad de error de medicación, calculando una tasa de erro de medicación por paciente, evaluando si una medicación de fuera del formulario impactaba en el potencial de error, determinando si un farmacéutico clínico disminuía la tasas de error de medicación, y evaluando si los pacientes que sufrían errores de medicación tenían más probabilidad de tener un tiempo de internamiento (LOS) mayor. Los análisis incluyeron estadística descriptiva, medias y correlaciones de Spearmen Rank.
Resultados: Hubo 344 pacientes incluidos en este estudio, 132 (38%) sufrieron 190 errores de medicación (1,44 errores/paciente). Una dosis omitida fue el error de ARV más frecuente y alcanzó un 30% (n=55) del total de errores. Hubo 166 pacientes que necesitaron medicación para IO, 37 de ellos sufrieron 39 errores de medicación. Omitir la profilaxis de IO contabilizó 31 errores. Sólo 45 de los 190 errores (24%) fueron corregidos antes del alta. La prescripción de al menos 1 medicamento fuera del formulario estaba correlacionado con aumento de errores (n=193 pacientes con medicamentos fuera de formulario, p<0,025, r=0,12). La actuación de un farmacéutico clínico no afectó al número de errores. Los pacientes que sufrieron un error tuvieron una LOS mayor (p=0,02).
Conclusiones: Los errores asociados a medicaciones ARV y para IO son frecuentes en pacientes infectados con VIH. Aparecen más errores en pacientes que reciben medicamentos fuera del formulario. Las intervenciones recomendadas incluyen la revisión del formulario, educación, y entrenamiento. Clínicos dedicados al VIH con entrenamiento adecuado y acreditados puede mejorar la gestión de esta enfermedad.
Palabras clave: Errores de Medicación; Agentes Anti-Retrovirales; Infecciones de VIH; Pacientes Internos; Estados Unidos.
Introduction
The number of individuals receiving combination antiretroviral therapy (cART) to manage Human Immunodeficiency Virus (HIV) and Acquired Immune Deficiency Syndrome (AIDS) has increased over the years as the CD4 threshold used to initiate cART has been broadened.1 Early cART effectively prolongs survival in this population of patients where HIV is now considered a chronic disease state. In fact, in 2015, 50% of HIV-infected individuals are projected to be above the age of 50.2 Therefore, it is important for providers in all settings to be well-versed on this chronic disease state management to ensure appropriate patient safety and efficacy of cART. Although cART has prolonged survival in patients with HIV/AIDS, medication errors may put these patients at risk for adverse events and even failure of cART since this population is more prone to medication errors due to transitioning to and from outpatient and institutional care (e.g. hospitals and correctional facilities).
In a 2014 review article evaluating 25 studies reporting HIV medication errors in the hospital setting, the incidence of errors was as high as 86% including both antiretroviral (ARV) and opportunistic infection (OI) prophylaxis medication errors.3 Additionally, a range of 1.16 - 2.7 medication errors per patient has been documented.4-6 Commonly identified errors were omission of an ARV5,7-9, inaccurate dosing frequency4-6,9-10, or drug-drug interactions.1,7 New ARVs continue to change the landscape of cART, but with these advancements, further potential for medication errors exist. Additionally, patients with more advanced HIV require extra medications for OI prophylaxis which provides another opportunity for medication errors.
The primary objective of this retrospective chart review was to assess the occurrence and type of ARV and OI medication errors within the inpatient setting in our institution. Secondary outcomes included assessing each ARV with an error and evaluating its potential risk for a medication error, calculating a medication error rate per patient, evaluating whether receipt of a non-formulary (NF) medication impacted the error potential, determining whether a clinical pharmacist on service reduced the medication error rate, and assessing length of stay (LOS) in patients who experienced a medication error.
Methods
This retrospective, observational, electronic medical chart review was conducted on inpatient HIV/AIDS patients admitted to our urban academic medical center. Inclusion criteria were HIV-infected patients with confirmed HIV/AIDS diagnosis, admitted to our institution between February 15, 2011 and May 22, 2012, at least 18 years of age, and actively taking cART and/or OI medications. We excluded patients not receiving or refusing ARV and/or OI medications, or newly diagnosed.
Collected baseline demographic data included gender, age, race, CD4 T-cell and HIV-RNA (viral load) at or within 3 months of admission, renal and/or hepatic impairment, and whether the patient was followed at our institution´s outpatient HIV clinics. Data regarding hospitalization included reason for admission, ARVs and OI medications (for prophylaxis and/or treatment) ordered, formulary status of ARV, date of admission, admitting medical service, presence of a clinical pharmacist on a medical service, presence of ID consult service, and LOS. All collected data was reviewed by the two study investigators with HIV specialty training.
Documented medication errors could occur at any time during the hospitalization and types of errors included an omitted or incomplete regimen, incorrect schedule or frequency, incorrect dose, missed dose(s), inappropriate renal dose adjustments, incorrect ARV ordered, and duplicate order. In addition to these potential medication errors, possible OI errors included inappropriate discontinuation or unnecessary prescribing of OI medications. Based on Micromedex 2.0® criteria, available in the institution during the observational period, "major" or "contraindicated" drug-drug interactions were collected and evaluated whether they were managed.11 Individual ARV and OI medications were compared to total number of ARV and/or OI errors, error types, whether or not the errors were corrected, and time to correction.
Each eligible patient´s electronic medical record (EMR) was reviewed by the investigators. Medication errors and drug interactions were evaluated through the patient chart and electronic medication administration record (EMAR) for the duration of the patient´s hospitalization. Appropriateness for dosing, scheduling, and dose adjustments were based on the 2012 Department of Health and Human Services (DHHS) HIV guidelines.1 The study received Institutional Review Board (IRB) approval in April 2012.
For our analysis, descriptive statistics were used to create tables of errors by medications. Spearman´s rank correlation was utilized to compare total number of errors to confounding variables. All statistical tests were conducted using SAS 9.2 (SAS Institution Inc., Cary, NC, USA). Statistical significance was set under a p-value of 0.05. Lastly, a two-sample t-test was used to compare the LOS between groups.
Results
Overall, 758 patient charts were reviewed with 344 patients included for analyses (Table 1 - baseline demographics, HIV-related admissions, and exclusion criteria). We found 190 medication errors occurred in 132 patients (mean 1.44 errors/patient, range of errors/patient 1-4). Table 2 shows overall ARV and OI medication errors and stratifies ARV and OI medication errors per patient. Only 45 (24%) overall medication errors were corrected within a median time of 36 hours (range 12-768 hours). Nearly half (n=22, 49%) of the 45 medication errors that were corrected had a clinical pharmacist on service. Clinical pharmacists most often identified omitted orders (13%) when correcting a medication error. The most common uncorrected medication errors when a clinical pharmacist was on service was also an omitted order (14%), dosing errors with raltegravir and lopinavir/ritonavir (8%), and scheduling errors with ritonavir (3%). There were 226 patients who received continuity of care at one of our institution´s outpatient HIV clinics. We found no association with our clinic patients experiencing an error compared to non-institution patients (n=76 patients, p=0.08, r=-0.121).
Looking specifically at ARV medications and errors, 320 patients received 103 different ARV regimens. One hundred thirteen (35%) of the 320 patients on cART experienced 151 errors (range 0-4 errors per patient, with 67% experiencing 1 error, 24% with 2 errors, 8% 3 errors and 1% with 4 errors ). Forty (26%) of these errors were corrected in a median time of 36 hours (range 12-768 hours). The most common ARVs prescribed with errors can be seen in Table 3. The most common ARV errors were omitted and/or incomplete order (n=57 errors, 38%) and incorrect dosing schedule (n=54, 36%) (Table 2). Examples of each included omitting a medication when splitting the single tablet regimen (STR) efavirenz/emtricitabine/tenofovir into its individual components, or when ritonavir was not being scheduled with the concomitant protease inhibitor (PI). The ARV classes listed in order of most errors were nucleoside reverse transcriptase inhibitors (NRTIs, n=61, 40% of ARV errors), protease inhibitors (PIs, n=48, 32%), non-NRTIs (n=21, 14%), integrase strand inhibitors (INSTIs, only raltegravir, n=20, 13%), and entry inhibitor (EI, n=1, <1%). We found no association between a specific ARV or class and a specific error type occurring.
Prescribing of NF medications was common as 193 patients (56%) were prescribed 259 NF medications (range 1-3 NF meds). The presence of at least 1 NF medication was correlated with increased errors (p<0.025, r=0.12). Most common NF medications included raltegravir (n=89), darunavir (400 or 600mg, n=46), abacavir/lamivudine (n=43), efavirenz/emtricitabine/tenofovir (n=42), and etravirine (n=25). All NF medications had a positive correlation error occurrence, except for efavirenz/emtricitabine/tenofovir (r=-0.11).
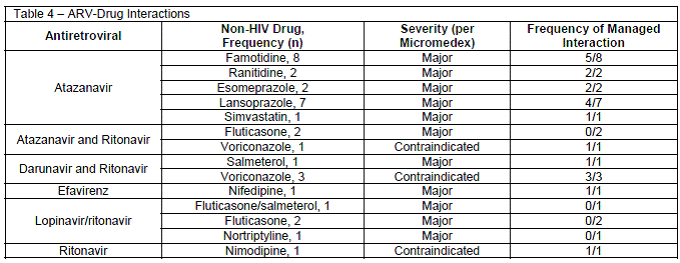
There were 32 patients (9%) who experienced 33 ARV-drug-drug interactions (Table 4). The most common interacting ARV was atazanavir having an interaction with either a histamine-2-receptor antagonist or proton-pump inhibitor (n=10 and n=9, respectively) as it related to the timing of administration. Twenty-one of the 33 drug-interactions (64%) were managed either by changing the interacting medication(s), monitoring in the clinical note or via laboratory markers, or a combination of these assessments. Ten of the 21 managed interactions occurred while a clinical pharmacist was on service. Of the 11 unmanaged interactions, 8 occurred on services with a clinical pharmacist. No association was found between drug-interactions, a specific medication or error type occurring, or clinical service.
There were 166 patients requiring OI medications (48% of the study population) resulting in 37 patients (22% of those receiving OI medications) experiencing 39 errors. Five (11%) OI errors were corrected in a median time of 24 hours (range 24-240). Table 2 reveals the breakdown of OI errors. We found no association between a specific OI or specific OI medication and a specific error type occurring.
The most common admitting service was the ID consult service (n=103, 30%), followed by general medicine (n=41, 12%). The ID consult service had a clinical pharmacist in training (either a post-graduate year 2 resident or infectious diseases pharmacotherapy fellow), on service for 31 of 103 (30%) admissions. Overall, 37 of the ID admissions encountered 55 medication errors (36% of ID admissions, 29% of overall errors, 1.49 medication errors/patient). The remaining 95 non-ID admissions encountered 135 errors (1.42 medication errors/patient). Nine of the 37 (24%) ID admissions with an error occurred while a clinical pharmacist was on service. Only 12 out of 55 (22%) errors were corrected while the training clinical pharmacist was on service.
A clinical pharmacist was present on any service for 168 of 344 (49%) admissions, with 64 patients experiencing 89 of the overall 190 medication errors (47% of patients, 1.39 errors/patient); thus, 101 errors from 176 admissions without a clinical pharmacist (53% of patients, 1.49 errors/patient). Based on this data, we found no difference regarding errors occurring with or without a clinical pharmacist on service (p=0.72, r=0.019) nor between ARV and OI errors occurring and admitting service.
Overall, when comparing LOS for patients who experienced or did not experience an error, we found that the average LOS for patients who experienced an error was 8.83 days/patient (1166 days/132 patients) compared to 6.67 days/patient without an error (1434 days/212 patients) (p=0.02). It is important to note that these results did not control for potential confounding variables such as admitting service (ICU vs. general medicine) or HIV status (well-controlled vs. AIDS), for example.
Discussion
Regardless of disease state, medication errors are unacceptable. The goal for all institutions should be no medication errors. The medication ARV and OI error rate per patient at our institution was 1.44, which is at the lower range of previously reported figures (1.16-2.7).5,6 The frequency of patients who experienced at least 1 ARV and OI medication error was 38%, which is near the middle of previously reported ranges, 2-86%4,5, and similar to the 35% recently reported by Commers and colleagues.12 It is difficult to compare ARV and OI medication errors from other studies due to the variability in study designs, interventions, data collection, and analysis.
Common errors in our study were omitted orders and/or incomplete regimens and incorrect dosing schedule. Omission of an ARV order was commonly found in other literature5,7-9,12, as was incomplete regimen.4,6-8,13 The frequency of dosing errors found in our study was similar to other studies as well.4-6,9,10 Our data revealed the highest frequency of errors occurred in the NRTI and PI classes. This is most likely attributable to the fact that the NRTIs are often the "backbone" of cART and frequently prescribed. The PI class has potential for complicated dosing and almost all require "boosting" with ritonavir, which can be confusing to those unfamiliar with these medications. These 2 classes have been frequently reported as the 2 most common ARV classes experiencing a medication error.4,5,10,12-14
Our study found drug interactions occurred in about 9% of patients, which is consistent with other reported literature.5,6,15 The most frequent errors occurred between atazanavir and acid-suppression medications. Overall interactions were appropriately addressed 64% of the time, regardless of whether a clinical pharmacist or ID trained provider was on service.
Comparing patients´ LOS between those who experienced an error and those who did not, our study found that those patients who experienced an error also experienced a 2.16 increased LOS in days. Although, this study did not look at outcomes, costs, and patient safety, this data may suggest potential cost-savings in preventing medication errors. Additionally, possible confounding variables were not controlled for in this assessment.
Non-Formulary Medications, Combination Tablets, and Single Tablet Regimens
The evolution of cART has created several STRs to decrease pill burden and increase medication adherence. This has been associated with better rates of virologic suppression.16 Compared to other NF medications, when a patient was on efavirenz/emtricitabine/tenofovir STR, they were less likely to experience an error. This could be due to the fact that this STR has a "1:1" conversion "splitting" efavirenz and emtricitabine/tenofovir both with the same doses and schedules. Also, providers may have been more familiar with that STR, as this was the first STR available on the market.
Similar to other literature, our review found that patients receiving at least 1 NF medication had an increased association of encountering an error.12,15,17 At the time of this review, all combination tablets (except: emtricitabine/tenofovir, lopinavir/ritonavir, and lamivudine/zidovudine) and the STR efavirenz/emtricitabine/tenofovir were considered NF and required "splitting" the tablet into individual formulary agents. It is important to note that not all of these have a "1:1" conversion when breaking into its individual components. In addition, no order sets for NF medications existed at our institution. This meant the provider had to place the required order with correct name/spelling, dose, and schedule of the medication without any prompts.
Importance of Appropriately Trained Clinicians
At our institution during the time of this review, an HIV-trained pharmacist was not on any inpatient service. For services that did have a clinical pharmacist present, medication reconciliation and verification occurred for each patient. Additionally, order entry was the responsibility of the admitting service or overnight medical resident. Furthermore, these orders may be entered when clinical pharmacy services were unavailable overnight or only through on-call paging services. However, every order was reviewed and processed by a staff pharmacist. Since our study identified 103 different ARV regimens in 320 patients, it is important to train and educate clinicians beyond basic HIV knowledge since there is great variability in what patients receive. Based on our evaluation, it appeared that providers and clinical pharmacists may have had minimal HIV education, training, or expertise in managing this specialized population.
In 2007, an electronic survey assessed ARV prescribing errors in 2 community teaching hospitals and concluded that physicians with no ID or HIV training were less knowledgeable regarding common ARV regimens and that ID or HIV trained specialists may reduce the risk of prescribing errors.18 Additionally, previous findings report a positive impact of clinical pharmacists in reducing ARV and OI medication errors, showing that 100% of clinical pharmacist recommendations were accepted and aided in decreasing the duration of those errors.13 In a correspondence to a Yehia and colleagues study, which reported 76% of medication errors documented were corrected within 48 hours, the authors stated that 2 clinical pharmacists who specialized in ID reviewed all medication orders for potential errors which may have contributed to the high percentage of medication errors corrected.13,19 These authors further stated that a clinical service such as the one demonstrated by Yehia and colleagues may be an appropriate model for other institutions to follow in order to prevent, minimize, and correct inpatient ARV medication errors.19 To further expand upon this idea, the importance of properly trained clinicians may be an avenue to develop an ARV stewardship program, similar to a multidisciplinary antimicrobial stewardship program reported by Sanders and colleagues which showed a decrease in ARV medication errors as well as improved resolution rate.15 This may suggest that only HIV-trained clinicians, including pharmacists and those with appropriate credentialing, should manage HIV patients. Additionally, as the paper by Li and Foisy states, the causes of errors are multifactorial. Some additional means of error prevention may include a pocket-card/quick reference for providers, training and education of staff, as well as systematic order entry.3 Furthermore, for institutions without specialized pharmacists, providers, or HIV services, a national HIV Telephone Consultation Service called Clinician Consultation Center may provide additional resources and assistance.20 Other potential interventions could be powerplans for admitted patients and a bi-annual formulary review, which may be essential as new ARVs and combination tablets are in the pipeline as well as changes to guideline recommendations.
ID Consult and the Impact of Clinical Pharmacy
At our institution, not all HIV-infected patients were followed by the ID consult service; only those admitted for an HIV-related diagnosis, those with unstable CD4 counts, and/or detectable viral loads. In addition, a clinical pharmacist, in HIV-training, was only on the ID consult service 30% of the time, compared to overall services with 49% clinical pharmacist coverage. Our data demonstrated that regardless of service and whether or not a clinical pharmacist was present errors occurred and were seldom corrected.
Although not found to be statistically significant, we found an increased medication error rate per patient followed by ID consult, which was opposite of previously reported findings.7,15,18 This may be attributed to inconsistent clinical pharmacy coverage, as there were fewer admissions with errors when a clinical pharmacist was on service, although this was also not found to be statistically significant. This information reinforces the necessity to have appropriately trained staff in an attempt to decrease errors and improve the number of errors corrected.15 Furthermore, when medication errors were corrected, there was little to no documentation stating whether it was due to a clinical or staff pharmacist intervention or recommendation which meant that even when errors were corrected, there was no way of knowing if this was secondary to a pharmacist. Lastly, a medication error correction may have been identified and communicated by a pharmacist, but was not corrected by the provider, even though appropriate measures were attempted.
Education, including systematic reviews and updates, is needed for clinical and hospital pharmacy, nursing, and ordering providers to familiarize the entire hospital staff with the complexity of ARV and OI medications, considering 320 patients accounted for 103 different cART regimens. Other developments include daily review by an HIV-trained and credentialed clinical pharmacist for medication reconciliation, discharge counseling, and coding ARVs as time-critical dosing for twice daily dosed medications at 09:00 and 21:00, as opposed to 09:00 and 17:00 (essentially, "q 12 hours vs. BID"). Corrigan and colleagues demonstrated that a pharmacist-driven medication reconciliation process was associated with a decreased error rate.10 Finally, an inpatient alert should be created to alert an HIV-trained clinical pharmacist practicing in the inpatient setting anytime an HIV-infected patient is admitted or anytime an ARV is ordered. This will allow for a formal review to identify and prevent ARV errors from occurring, with all recommendations being passed onto the necessary clinician.13
Limitations
Limitations to our study included inconsistent EMAR documentation which may have altered the true number of errors documented due to the retrospective nature of our study. Previous literature reported discrepancies between medication administration and EMAR documentation, claiming that medications were administered to the patient without being documented in the EMAR and vice-versa.21 Furthermore, as clinical pharmacists were not on every service and infrequently provided notes in the EMR, it is unknown whether their recommendations to correct an error or interaction were not made at all or if their recommendations were not accepted. Similarly, some medication errors may have been corrected staff by pharmacists, which also went undocumented. Therefore, it is difficult to assess the true impact of clinical and hospital pharmacy services within our study. Lastly, being a retrospective design may have provided less detail with respect to the errors, timing, and types compared to a prospective design.
Conclusions
The overall goal is to have no medication errors, and if an error occurs, to have it corrected quickly. At our institution, ARV and OI medication errors occurred at frequencies comparable to previously reported studies. A major variable influencing medication errors included taking a NF medication. We observed that documented correction of medication errors for the clinical specialist was approximately 50%. Although omitted orders were the most corrected error by clinical pharmacists, it also accounted for the most uncorrected error type as well. The "3-active drug" requirement for ART was not always upheld because 38% of the time, an ARV was omitted leading to an incomplete regimen. This could lead to the loss of virologic suppression, development of resistance, as well as the patient continuing the wrong regimen when released from the hospital. It is essential to perform accurate medication reconciliation upon hospital admission and discharge. Based on our findings, dedicated HIV clinicians with adequate training and credentialing may improve the management of this specialized disease state as they are more familiar with ARV and OI medications. Additional recommendations to improve error rates include having an open ARV formulary with appropriate order sentences, bi-annual formulary review/update, increased staff education, including systematic order entry and verification for providers, pharmacists, and nurses, and a systematic medication review by an HIV-trained pharmacist. Future studies are necessary to monitor patient outcomes as a result of medication errors as well as cost-savings from decreased LOS.
Acknowledgements
Rodrigo Burgos, PharmD, AAHIVE; Larry H. Danziger, PharmD, FIDSA; Andrew J. Donnelly, PharmD, MBA, FASHP; Siyun Liao, PharmD, PhD, BCPS; Renata Smith, PharmD, AAHIVP.
Conflict of interest
There are no conflicts of interests to report for any authors. This information has not been submitted anywhere else.
References
1. Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 1-239. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf (Accessed March 30, 2012). [ Links ]
2. P.I. Perspective: 25 years of information, inspiration and advocacy for people living with HIV/AIDS. Project Inform Web site. http://img.thebody.com/pinf/2010/pip50.pdf#page=7 (Accessed June 21, 2013). [ Links ]
3. Li EH, Foisy MM. Antiretroviral and medication errors in hospitalized HIV-positive patients. Ann Pharmacother. 2014;48(8):998-1010. [ Links ]
4. Purdy BD, Raymond AM, Lesar TS. Antiretroviral prescribing errors in hospitalized patients. Ann Pharmacother. 2000;34(7-8):833-838. [ Links ]
5. Snyder AM, Klinker K, Orrick JJ, Janelle J, Winterstein AG. An in-depth analysis of medication errors in hospitalized patients with HIV. Ann Pharmacother. 2011;45(4):459-468. doi: 10.1345/aph.1P599. [ Links ]
6. Rastegar DA, Knight AM, Monolakis JS. Antiretroviral medication errors among hospitalized patients with HIV infection. Clin Infect Dis. 2006;43(7):933-938. [ Links ]
7. Mok S, Minson Q. Drug-related problems in hospitalized patients with HIV infection. Am J Health Syst Pharm. 2008;65(1):55-59. [ Links ]
8. Heelon M, Skiest D, Tereso G, Meade L, Weeks J, Pekow P, Rothberg MB. Effect of a clinical pharmacist´s interventions on duration of antiretroviral-related errors in hospitalized patients. Am J Health Syst Pharm. 2007;64(19):2064-2068. [ Links ]
9. Rao N, Patel V, Grigoriu A, Kaushik P, Brizuela M. Antiretroviral therapy prescribing in hospitalized HIV clinic patients. HIV Med. 2012;13(6):367-371. doi: 10.1111/j.1468-1293.2011.00977.x. [ Links ]
10. Corrigan MA, Atkinson KM, Sha BE, Crank CW. Evaluation of pharmacy-implemented medication reconciliation directed at antiretroviral therapy in hospitalized HIV/AIDS patients. Ann Pharmacother. 2010;44(1):222-223. doi: 10.1345/aph.1M052. [ Links ]
11. Micromedex Healthcare Series. DRUGDEX System. Greenwood Village, CO: Truven Health Analytics, 2013. http://www.thomsonhc.com/. [ Links ]
12. Commers T, Swindells S, Sayles H, Gross AE, Devetten M, Sandkovsky U. Antiretroviral medication prescribing errors are common with hospitalization of HIV-infected patients. J Antimicrob Chemother. 2014;69(1):262-267. doi: 10.1093/jac/dkt323. [ Links ]
13. Yehia BR, Mehta JM, Ciuffetelli D, Moore RD, Pham PA, Metlay JP, Gebo KA. Antiretroviral medication errors remain high but are quickly corrected among hospitalized HIV-infected adults. Clin Infect Dis. 2012;55(4):593-599. doi: 10.1093/cid/cis491. [ Links ]
14. Pastakia SD, Corbett AH, Raasch RH, Napravnik S, Correll TA. Frequency of HIV-related medication errors and associated risk factors in hospitalized patients. Ann Pharmacother. 2008;42(4):491-497. doi: 10.1345/aph.1K547. [ Links ]
15. Sanders J, Pallotta A, Bauer S, Sekeres J, Davis R, Taege A, Neuner E. Antimicrobial stewardship program to reduce antiretroviral medication errors in hospitalized patients with human immunodeficiency virus infection. Infect Control Hosp Epidemiol. 2014;35(3):272-277. doi: 10.1086/675287. [ Links ]
16. Nachega JB, Parienti JJ, Uthman OA, Gross R, Dowdy DW, Sax PE, Gallant JE, Mugavero MJ, Mills EJ, Giordano TP. Lower pill burden and once-daily dosing antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;58(9):1297-1307. doi: 10.1093/cid/ciu046. [ Links ]
17. Kelly LS, Caulder CR, Bookstaver PB. Timely formulary management for preventing errors related to antiretroviral drugs. Am J Health Syst Pharm. 2013;70(12):1014-1015. doi: 10.2146/ajhp130078. [ Links ]
18. Arshad S, Rothberg M, Rastegar DA, Spooner LM, Skiest D. Survey of physician knowledge regarding antiretroviral medications in hospitalized HIV-infected patients. J Int AIDS Soc. 2009;12:1. doi: 10.1186/1758-2652-12-1. [ Links ]
19. Holtzman CW, Gallagher JC. Antiretroviral Medication Errors Among Hospitalized HIV-Infected Adults. Clin Infect Dis. 2012;55(11):1585-1586. doi: 10.1093/cid/cis721. [ Links ]
20. Clinician Consultation Center. HIV/AIDS Management Web site. Available at http://nccc.ucsf.edu/clinician-consultation/hiv-aids-management/ (Accessed November 21, 2014). [ Links ]
21. Max BE, Itokazu G, Danziger LH, Weinstein RA. Assessing unit dose system discrepancies. Am J Health Syst Pharm. 2002;59(9):856-858. [ Links ]
Received (first version):09.09.14
Accepted: 04.01.15