INTRODUCTION
Substandard and falsified medicines constitute a global challenge for health systems, especially in low-to-middle income countries (LMIC). A recent meta-analysis reported a prevalence of 13.6% of substandard and falsified medicines in LMIC.1 World Health Organization estimated that about one in 10 medical products in LMIC are substandard and falsified products.2 substandard and falsified medicines burden health systems, erode confidence in the system, increase the risk of illness morbidity and mortality. Substandard and falsified medicines waste essential drugs earmarked by government and individuals, which LMIC residents often cannot afford.1,2
Nigeria is reported to have one of the highest incidences of substandard and falsified medicines in Sub-Saharan Africa.3 Over the years, the estimated prevalence of substandard and falsified medicines seems to have reduced in Nigeria from 67% in 2001 to 5% in 2012.3 Also, there have been several reports of fatalities from the consumption of substandard and falsified medicines in Nigeria.4 Consequently, the Nigerian medicine regulatory authority, National Agency of Food Drug Administration and Control (NAFDAC), adopted multiple approaches to combat the problem of substandard and falsified medicines. Some of the approaches include deployment of product authentication devices like TRUSCAN® for in-the-field test for authenticity of product, retailer product authentication using radio frequency identification tagging and the Mobile Authentication Service (MAS).3,5 MAS involves consumers’ use of mobile phones to verify the source of medicines at the point of purchase at medicine retail outlets.
MAS is a mobile health technology (mHealth) deployed to hinder the retailing of falsified medicines to consumers. The service is provided by NAFDAC-approved, third party private information-communication technology firms (MAS providers) to pharmaceutical manufacturers and importers in Nigeria. According to a recent NAFDAC implementation guideline, marketing authorisation holder of any medicines seeking to engage the MAS providers’ services must notify NAFDAC and MAS providers must verify the NAFDAC registration status of medicines before deployment. NAFDAC should receive safe and secure data about deployed MAS from service providers.6
Pharmaceutical manufacturers or importers enabled their product packs through MAS service providers. The MAS providers grant pharmaceutical companies access to MAS scratch panel, which is affixed to the product. The scratch panel covers an alphanumeric code, which is a one-time use product personal identification number (PIN). Consumers are expected to scratch the panel and text the PIN to a dedicated phone number provided on the medicine pack. The consumer should receive a real-time, short message service (SMS) text response to authenticate the source of the medicine. Following request by consumers, all MAS providers now have a unified response which is either; a ‘confirmed’ message, to indicate that the drug product is genuine, or ‘unregistered product’, message, to show that the originality of the product cannot be verified. In this latter case, the consumer should call up the contact centre listed on the drug product for further directive.6 In 2012, NAFDAC rolled out the mandatory first phase of MAS deployment on all antimicrobials and anti-malaria medicines in Nigeria.7
A study reported that MAS responses positively correlate with the quality of medicine products and another small scale study reported that manufacturer/importer found the deployment of MAS effective to curb falsification of their products.8,9 However, there are reports suggesting there are challenges with the use of MAS in Nigeria. Several studies have documented underutilization by consumers despite a reasonable level of awareness.10,11,12 A pilot study demonstrated that response was not always real-time.13 Also, some community pharmacists reported that MAS impacted their practices negatively. These community pharmacists complained that response is not always real time, as expected, and consumers sometimes get no or wrong responses to MAS queries.14 These points-of-failure of the MAS have inadvertently portrayed the affected community pharmacies as sources of substandard and falsified medicines. Therefore, the acceptance of MAS among community pharmacists is unknown. Acceptance of MAS by community pharmacists is important for MAS to remain relevant as a tool for tracking substandard and falsified medicines in retail pharmacy practice. In addition, the challenges encountered by MAS providers at the point of deployment, which might contribute to these points of failures, are also unknown. For MAS continued usefulness, challenges associated with its deployment need investigation.
The objectives of this study were (1) to assess the acceptance of MAS by community pharmacists; (2) to explore the views of MAS providers about the challenges and successes of MAS deployment in Nigeria.
METHODS
Study design
A mixed-methods approach was employed to investigate the stated objectives. A quantitative cross sectional survey was used to investigate community pharmacists’ acceptance of MAS, while a qualitative research approach was employed to explore MAS providers’ views of challenges and successes of its deployment in Nigeria.
Ethics Approval
The study protocol was approved by the Lagos University Teaching Hospital Health Research Ethics committee (Assigned no: ADM/DCST/HREC/APP/1801). The protocol was, however, exempted from a full review.
Study population
The study population for the acceptance of MAS by community pharmacists consists of some nationwide community pharmacists registered with the Pharmacists’ Council of Nigeria. NAFDAC-approved MAS providers in Nigeria were recruited to explore their views on MAS deployment.
Study Instruments Development
Two study instruments were developed, standardized and used for the study. Firstly, a validated structured questionnaire for the community pharmacists (Cronbach alpha=0.949) was developed based on the Technology Acceptance Model (TAM).15 The instrument was used to collect demographics of participants and their responses to questions based on the model constructs (online Appendix). TAM model was selected for the study because reviews have found it to be robust and versatile to predict technological acceptance and usefulness in a wide variety of context.16 The model postulates a causal relationship between users’ perception of a technology and their acceptance of the technology and between their acceptance and use.17
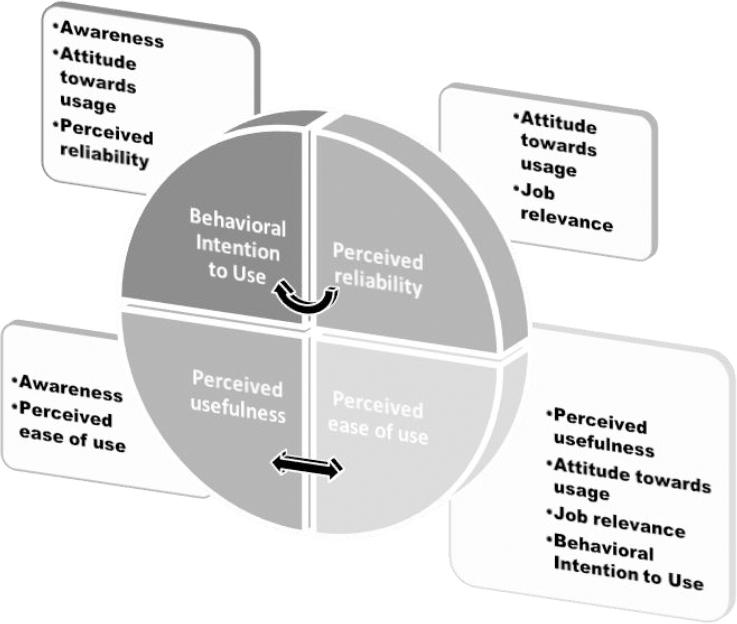
TAM is built on two independent variables; perceived usefulness and perceived ease of use; and the dependent variable: attitude towards use. According to the model, a user’s perceptions about a system’s usefulness and ease of use results in a behavioural intention to use (or not to use) the system. In order to meet the objectives of this research, the TAM was extended to include awareness, job Relevance and perceived reliability to help understand and explain behavioural intention to use MAS.
Secondly, a structured interview guide was developed to assess three domains of MAS providers’ views. These are successes of MAS deployment, the challenges with the deployment of MAS, as well as possible action plans for pharmacists when it fails (no response to their customers’ queries). These domains were based on personal communications with community pharmacists, previous study with manufacturers that have deployed MAS and reports in Nigeria newspapers.
Sample size and sampling technique
An online sample size calculator (Raosoft®) was used to calculate study sample size.18 At 95% confidence level, 5% margin of error and a total of 5435 registered community pharmacists (2016 register of Pharmacists Council of Nigeria), the study sample was 359. A response rate of 50% was assumed, therefore, a total of 573 questionnaires were planned for distribution. The study used convenience sampling method. Pharmacists that visited zonal offices of Pharmacists’ Council of Nigeria, across the country during the study period, were introduced to the study and offered the questionnaire to fill and return, as soon as possible.
For the qualitative exploration, all service providers were invited to participate in the study. There were five NAFDAC-approved service providers as of the time of the study. However, only three providers participated in the study.
Data collection
The study questionnaires, with a brief introduction, were distributed to community pharmacists through the Pharmacists’ Council of Nigeria zonal offices nationwide. Informed consent was assumed, if questionnaire is returned back to the zonal offices.
The five NAFDAC-approved service providers were contacted through the official phones available on their websites. They were offered two options of interview media: face-to-face interview or by e-mail. The e-mail is a viable and valuable medium for qualitative study interview though with limitations.19 All the service providers opted for the e-mail option and provided the researcher with appropriate e-mail addresses of the personnel that responded. The structured interview guide was then sent to and received from service providers by e-mails. Reminder e-mails were sent twice to the two unresponsive MAS providers, but their continued non-response was assumed to indicate their unwillingness to participate in the study.
Data analysis
All the statistical analysis in this section was done using R.20 Demographic analysis was carried out by computing descriptive statistics. To assess the internal consistency of the constructs, reliability and validity were measured using the Cronbach’s alpha. Hypotheses were formed to investigate causality/dependencies between the constructs. Multiple linear regression models were built on each set of hypotheses and the model diagnostics were examined to validate each hypothesis.
The textual nature of e-mail exchanges allows for thematic analysis, which was used to analyse returned MAS provider’s questionnaires.19 The concepts that were explored were predetermined, as covered by the structured interview guide sent to providers. Concepts explored include success, challenges, lack of response to authentication queries, possible pharmacists’ responses and the future of MAS in Nigeria. Codes were identified from the collected data and mapped into the predetermined concepts to form the coding framework.
RESULTS
The results are presented in two parts. In the first part, the results of the quantitative cross sectional survey of the community pharmacists were presented. The second part reported the findings of qualitative study of the MAS service providers.
Part A
A total of 326 community pharmacists filled and returned their questionnaires, which were analysed to serve as the sample size. The results showed that over 67% of respondents were males (Table 1). Furthermore, about half (about 46%) of the respondents had less than 5 years community experience, while less than 1% of the respondents had more than 40 years community pharmacy practice experience.
Table 1. Descriptive statistics of respondents’ characteristics (n=326)
| Items | Frequency | Percent |
|---|---|---|
| Age | ||
| Less than 25 | 14 | 4.29 |
| 25-30 | 83 | 25.46 |
| 31-40 | 115 | 35.28 |
| 41-50 | 68 | 20.86 |
| above 50 | 46 | 14.11 |
| Gender | ||
| Male | 218 | 67.08 |
| Female | 107 | 32.92 |
| Years of community pharmacy practice | ||
| Less than 5 | 151 | 46.89 |
| 6-10 | 74 | 22.98 |
| 11-20 | 54 | 16.77 |
| 21-30 | 25 | 7.76 |
| 30-40 | 17 | 5.28 |
| Above 40 | 1 | 0.31 |
Table 2 shows the descriptive statistics of the variables in the study (i.e. Awareness, Perceived usefulness, Perceived ease of use, Attitude towards usage, Perceived reliability, Job relevance and Behavioural Intention to use). The reliability of each variable was computed using Cronbach Alpha.20 Apart from “Perceived ease of use”, which had a reliability coefficient of 69%, all independent variables demonstrate acceptable values (>0.70), which indicates that they were reliable measures for their respective constructs. Table 3 below documented the list of hypotheses considered in investigating causality/dependencies between the constructs. Multiple linear regression models were built on each set of hypotheses and the model diagnostics were examined to validate each hypothesis. The results of the model diagnostics are presented in Table 4. Figure 1 gives a summary of causality/dependencies between the constructs based on the supported hypothesis in Table 4.
Table 2. Mean, standard deviation and alpha coefficient of study variables (n=326)
| Variable | Mean | SD | Alpha |
|---|---|---|---|
| Awareness | 1.26 | 0.42 | 0.72 |
| Perceived usefulness | 3.31 | 0.85 | 0.74 |
| Perceived ease of use | 3.86 | 0.71 | 0.69 |
| Attitude towards usage | 2.98 | 0.8 | 0.71 |
| Perceived reliability | 2.73 | 0.82 | 0.70 |
| Job relevance | 6.96 | 0.89 | 0.73 |
| Behavioral Intention to Use | 3.59 | 0.98 | 0.79 |
SD: Standard deviation
Table 3. Hypothesis considered
| H1a | Behavioral intention to use the MAS is positively influenced by awareness. |
| H1b | Behavioral intention to use the MAS is positively influenced by job relevance. |
| H1c | Behavioral intention to use the MAS is positively influenced by attitude towards usage. |
| H1d | Behavioral intention to use the MAS is positively influenced by perceived ease of use. |
| H1e | Behavioral intention to use the MAS is positively influenced by perceived usefulness. |
| H1f | Behavioral intention to use the MAS is positively influenced by perceived reliability. |
| H2a | Attitude towards MAS usage is positively influenced by awareness. |
| H2b | Attitude towards MAS usage is positively influenced by job relevance. |
| H2c | Attitude towards MAS usage is positively influenced by behavioral intention to use MAS. |
| H2d | Attitude towards MAS usage is positively influenced by perceived ease of use. |
| H2e | Attitude towards MAS usage is positively influenced by perceived usefulness. |
| H2f | Attitude towards MAS usage is positively influenced by perceived reliability. |
| H3a | Perceived usefulness of MAS is positively influenced by awareness. |
| H3b | Perceived usefulness of MAS is positively influenced by job relevance. |
| H3c | Perceived usefulness of MAS is positively influenced by attitude towards usage. |
| H3d | Perceived usefulness of MAS is positively influenced by behavioral intention to use MAS. |
| H3e | Perceived usefulness of MAS is positively influenced by perceived ease of use. |
| H3f | Perceived usefulness of MAS is positively influenced by perceived reliability. |
| H4a | Perceived ease of use of MAS is positively influenced by awareness. |
| H4b | Perceived ease of use of MAS is positively influenced by job relevance. |
| H4c | Perceived ease of use of MAS is positively influenced by attitude towards usage. |
| H4d | Perceived ease of use of MAS is positively influenced by behavioral intention to use MAS. |
| H4e | Perceived ease of use of MAS is positively influenced by perceived usefulness. |
| H4f | Perceived ease of use of MAS is positively influenced by perceived reliability. |
| H5a | Perceived reliability of MAS is positively influenced by awareness. |
| H5b | Perceived reliability of MAS is positively influenced by job relevance. |
| H5c | Perceived reliability of MAS is positively influenced by attitude towards usage. |
| H5d | Perceived reliability of MAS is positively influenced by behavioral intention to use MAS. |
| H5e | Perceived reliability of MAS is positively influenced by perceived ease of use. |
| H5f | Perceived reliability of MAS is positively influenced by perceived usefulness. |
Table 4. Results of regression tests. Each test reports dependent variable first, followed by the list of independent variables beta.
| Regression test | Adjusted R2 | beta | Hypothesis result |
|---|---|---|---|
| Behavioral intention to use | 0.17*** | ||
| Awareness | 0.30* | H1a: supported | |
| Job relevance | 0.01 | H1b: not supported | |
| Attitude towards usage | 0.23** | H1c: supported | |
| Perceived ease of use | 0.14 | H1d: not supported | |
| Perceived usefulness | 0.12 | H1e: not supported | |
| Perceived Reliability | 0.29*** | H1f: supported | |
| Attitude towards usage | 0.15*** | ||
| Awareness | -0.18 | H2a: not supported | |
| Job relevance | 0.19 | H2b: not supported | |
| Behavioral intention to use | 0.18** | H2c: supported | |
| Perceived ease of use | 0.23*** | H2d: supported | |
| Perceived usefulness | 0.14 | H2e: not supported | |
| Perceived Reliability | -0.07 | H2f: not supported | |
| Perceived usefulness | 0.18*** | ||
| Awareness | 0.24** | H3a: supported | |
| Job relevance | 0.00 | H3b: not supported | |
| Behavioral intention to use | 0.07 | H3c: not supported | |
| Attitude towards usage | 0.05 | H3d: not supported | |
| Perceived ease of use | 0.23*** | H3e: supported | |
| Perceived reliability | 0.09 | H3f: not supported | |
| Perceived ease of use | 0.21*** | ||
| Awareness | -0.20 | H4a: not supported | |
| Job relevance | 0.27** | H4b: supported | |
| Behavioral intention to use | 0.20*** | H4c: supported | |
| Attitude towards usage | 0.10 | H4d: supported | |
| Perceived usefulness | 0.38*** | H4e: supported | |
| Perceived reliability | -0.10 | H4f: not supported | |
| Perceived reliability | 0.14*** | ||
| Awareness | 0.19 | H5a: not supported | |
| Job relevance | 0.32** | H5b: supported | |
| Behavioral intention to use | -0.07 | H5c: not supported | |
| Attitude towards usage | 0.21*** | H5d: supported | |
| Perceived ease of use | 0.16 | H5e: not supported | |
| Perceived usefulness | -0.10 | H5f: not supported |
Dependent variables are represented in bold italics
*p<0.1
**P<0.05
***p<0.01
It was observed from Table 4 that hypothesis H1a, H1c and H1f were supported indicating that awareness, attitude and perceived reliability all positively affect behavioural intention to use MAS.
Also, hypothesis H2c and H2d were supported so that only behavioural intention to use MAS and perceived ease of use have positive effect on attitude to MAS usage. It was also observed that awareness and perceived reliability negatively affect attitude to use MAS.
Hypothesis H3a and H3e were supported indicating that awareness and perceived ease of use positively affected perceived usefulness of MAS. Hypothesis H4b, H4c, H4d, H4e were supported, indicating that job relevance, behavioural intention to use, attitude towards usage and perceived usefulness all positively affected perceived ease of use, while awareness and perceived reliability negatively affected perceived ease of use. Furthermore, hypothesis H5b and H5d were supported. It can be inferred that only job relevance and attitude towards usage positively affected perceived reliability, but behavioural intention to use and perceived usefulness negatively affected perceived reliability
It was also observed from Table 4 that awareness (beta=0.30) and perceived reliability (beta=0.29) were the highest influencers of behavioural intention to use MAS, while perceived ease of use (beta=0.23) was the highest influencer of attitude towards MAS usage.
Again, awareness (beta=0.24) and perceived ease of use (beta=0.23) were the highest influencers of perceived usefulness and perceived ease of use (beta=0.38) was the highest influencer of perceived usefulness. In addition, Job relevance (beta=0.32) was the highest influencer of perceived reliability.
Furthermore, the result showed that more than half (53%) of respondents were keen on using MAS, 51% of respondents reported that they would recommend the service to other practitioners and 54% indicated they would encourage their clients to use the service. It was also observed that 46% would check the authenticity of all medication before dispensing, while 48% would only check if the medication is from an unfamiliar source. Overall, it can be inferred that about half of the community pharmacists in our coverage regions were positively disposed towards the use of MAS.
Part B
The findings on the views of MAS providers presented in a 3-part section covered the successes and challenges of MAS deployment in Nigeria. The first section presented the themes that captured MAS providers’ views of the successful deployment. The second section highlighted themes that expressed the challenges encountered during deployment, which might be responsible for pharmacists experiences of no or wrong responses to queries. The final section explained MAS providers’ views on what pharmacists can do when MAS queries give no or wrong response.
Theme: Circumstantial evidence to support success of MAS
Service providers seem to view the deployment of MAS as successful tool to prevent sales of MAS-enabled substandard and falsified medicines. Their views were based on increase in sales of MAS-enabled medicines by their manufacturers/importers. Also, service providers expressed the adoption of MAS by pharmaceutical companies, whose products are not required by regulations to use MAS, as a testament of the success of the technology.
SP1: “...Pharmaceutical companies are seeing an increase in sales after implementation of the technology...”
However, a service provider expressed lack of objective evidence to support success of MAS technology.
SP2: “Success is reduction in the prevalence of fake drugs as a result of deployment of the technology. There is no empirical study to test that claim yet. Therefore, I cannot tell if it is a success yet.”
The current measures of MAS success by service providers appear subjective.
Theme: Lack of responses to MAS queries are due to factors in the setting
The main challenge reported by pharmacists was no or wrong response to MAS queries by consumers. The factors that contributed to this challenge were mainly contextual to the Nigerian setting. In this study, three major contextual challenges were described. Firstly, was the global system mobile (GSM) phone operation in Nigeria.
SP1: “...the telecom providers having downtime with their service, which affects prompt delivery of the messaging. If a consumer has also ported their lines, it takes a while for the system to search for the right network to send the message...”
Secondly, MAS providers’ equipment are affected by the perennial power outages in Nigeria, which further compounds the no-response-to-queries challenge. Thirdly, the proficiency of consumers to use SMS facility on their phone may be low, which contributes to the wrong response challenge.
SP3: “Issue of the customers sending the wrong pin, as inscribed on the labels thereby prompting error message.”
Theme: Fragile business model of MAS provision
Another contributory factor to poor responses to MAS queries may be the current business model for service provision, which appears fragile. Two subthemes emerged for this theme. Firstly, the MAS queries and responses operate on existing GSM infrastructure. The MAS service providers appear to incur enormous cost for using this infrastructure, as it seems that the current business model of the MAS platform yields profit when less than 5% of the codes are queried.
SP2: “It is a good technology. However, the economics of the technology is only profitable if less than 5% of codes are queried...”
In addition, the service providers recognise huge telecommunications costs as a challenge, which may have contributed to the inefficiency of their services.
The second subtheme is when pharmaceutical companies may affix MAS codes on distributed medicines packs without activating them by service providers.
SP3: “The issue of non-activation of code by the service providers before taking it to the market by the pharmaceutical companies”
The fragile business model of service providers might have contributed to the inefficiency of MAS in Nigeria.
Theme: The future: Community pharmacists as MAS champions
To overcome the challenge of no or wrong response, MAS providers described other technological platforms, call centres and web applications, which pharmacists could assess to query their codes. However, these applications might shift cost of sending MAS queries to the consumers rather than to service providers, which may probably boost profits.
SP1: “In the event that there is no response to the message inquiring about authenticity, it would be best if the pharmacist recommends other channels of verification like placing a phone call to the call centre, verifying through the app or the website. These other channels are just as reliable”
There may be need to engage community pharmacists, as MAS champions, in its future deployment to promote its use on all MAS platforms – SMS, call centres and web applications.
DISCUSSION
This study employs TAM models, modified to include awareness and perceived reliability to help understand and explain behavioural intention of the community pharmacists to use MAS. One of the key findings of this research was that just about half (53%) of the respondents community pharmacists were keen on MAS. This is not surprising because the initial deployment of MAS did not include specific codes for pharmacists’ verifications. MAS was designed strictly for consumers to authenticate purchased products. The omission of pharmacists’ codes made MAS different from SecurPharm®, which is a pharmacist-driven authentication process.22 SecurPharm implementation in Germany makes it obligatory for pharmacists to authenticate every medicines before dispensing, for MAS, consumers could not be obligated to use it.22 A study noted that getting pharmacists’ to authenticate medicines may actually improve use of MAS by consumers.8 The recent revision of MAS implementation guideline seems to have incorporated pharmacists in the MAS process.6
The results of the study indicated that both awareness and perceived reliability played an important role in the behavioural intention to use MAS. However, no study was identified that have looked at acceptance of MAS in Nigeria or other climes. Therefore, to improve community pharmacists’ MAS acceptance, the National medicine regulatory authority and MAS providers may need to work on awareness and reliability of MAS.
In addition, it was observed that perceived ease of use is one of the strongest influencers of perceived usefulness. This is consistent with past studies involving TAM constructs i.e. an individual, who perceives a system to be easy to use, is more likely to perceive the system to be useful.21
In order to increase the number of community pharmacists who are positively disposed to use MAS, we would recommend a more aggressive MAS awareness campaign, which focuses on its reliability and ease of use.
The findings of the exploration of MAS providers’ views revealed that MAS, a complex intervention, appears to have been impacted by the local context, where it was implemented, to produce challenges that may have limited its effectiveness. The context, where a complex intervention is deployed is crucial and may play a role in the success of the intervention.22,23 The MAS technology is dependent on sending and receiving short message service (SMS) through GSM phones. Therefore, quality of service of the phone companies has direct effect on quality of MAS provided. In Nigeria, though subscription to GSM services is relatively high, 55 million subscribers in 1997, the GSM service efficiency is middling.24,25 The service efficiency of GSM is plagued by contextual challenges, like instability in power supply, lack of secured infrastructure and technical problems.24,25 These challenges have impacted MAS deployment in Nigeria.
The success, or not, of MAS to reduce circulation of falsified medicine is currently difficult to quantify because several interventions were implemented about the same time by the regulatory body.3,5 MAS deployment would have benefited from implementation theories, design of measurable outcomes and process evaluation framework from the initiation, as recommended by the Medical Research Council guidance.22 The process evaluation framework would identify measurable objective outcomes, which would highlight the mechanisms by which MAS reduces counterfeiting. It is important to explore mechanisms of how interventions, like MAS, bring change or not. This is crucial to understand both how the effects of the specific intervention occurred and how these effects might be replicated by similar future interventions.26 As NAFDAC considers future deployment, it is important for appropriate framework to be in place to evaluate its impact on counterfeiting.
Successful implementation of technological innovations is closely linked with champions that may actively promote, educate, advocate and make necessary connection between people for the innovations.27,28 Pharmacists, as champions, were considered crucial in overcoming some barriers to implementation of pharmacy bar codes scanning system in a hospital.29 Community pharmacists, who are often present at the point of purchase, can actively promote MAS and help consumers navigate the different platforms of MAS queries. This will enhance awareness of consumers about substandard and falsified medicines, as well as improve the use of MAS. This possibility of enhanced use of MAS was reported in a study of MAS.8
Substandard and falsified medicines impact community pharmacies’ practices negatively, and success of MAS deployment might benefit them, as its efficiency improves. However, the current format of MAS deployment seems to bypass community pharmacists, who have no means of product authentication via MAS platform prior to the time their client use it before payment. So they are often unprepared when clients’ authentication attempts fail. Going forward, NAFDAC may need to develop appropriate interventions that will improve MAS acceptance among community pharmacists, as well as involve them as MAS champions.
Limitations
The non-probability sampling methods limit generalization from the study. However, the national coverage for data collection and extant literature support that acceptance of MAS by community pharmacists may not be very high. The use of e-mails, as a means of interview, did not give room for prompt follow-up questions and response is dependent on respondents’ ability to write effectively. This, therefore, may have limited the richness of information obtained.
CONCLUSIONS
Acceptance of mobile authentication service by community pharmacists is moderate. The positive influencers of behavioural intention to use MAS are perceived reliability and awareness. Improving community pharmacists’ experiences with MAS by involving them in the verification process, as part of their professional obligations, might be crucial. The limitations for MAS deployment from MAS providers’ views are mainly contextual: downtime of GSM, power outages and limited ability of consumers’ to use SMS. This limitation may be mitigated by getting pharmacists, as MAS champions, to help consumers’ navigate other platforms like call centres and web applications for product authentication.