INTRODUCTION
Monitoring hospital antimicrobial use and resistance is key to antimicrobial stewardship (AMS) efforts to curtail the rise of antimicrobial resistance. AMS programs monitor compliance with interventions that aim to optimise therapy and identify utilisation patterns that warrant further investigation. In many countries hospital-level data also contribute to large-scale surveillance programs that enable benchmarking and epidemiological research.1
In the absence of patient-level data (typically from electronic prescribing or medication administration systems) to capture actual days of therapy (DOT) and prescribed daily doses, antimicrobial use in adult hospitals is frequently sourced from pharmacy information systems and reported using the defined daily dose (DDD). These numerators are standardised by a measure of activity (denominator) such as patient days or admissions. The World Health Organization’s DDD is considered a technical unit of measure based on the average daily dose of each agent for its most common indication in an adult. As the DDD is a fixed value, it cannot account for variations attributed to individual patient requirements such as indication, age or weight-based dosing or dose adjustment for comorbidities such as renal impairment.2 For this reason, DDD is not validated or endorsed for use in paediatric populations where individualised prescribing is commonplace.3 As a consequence, hospital utilisation data for paediatric patients may be excluded from larger antimicrobial surveillance programs that rely on DDD to monitor use in hospitals and the community.1,4
Despite these limitations, AMS programs in children’s hospitals without access to patient level records are expected to monitor antimicrobial usage patterns, and demonstrate cost-effective antimicrobial therapy.5,6 Surveys of actual prescribing, though ideal, are resource intensive in the absence of electronic systems and may not be feasible for routine surveillance. Therefore, pharmacy information systems continue to be used for antimicrobial utilisation using measures such as drug costs, DDD and paediatric defined daily doses.7
In the absence of any endorsed paediatric measure for hospitals without access to patient level data, The WHO recommend prescribed doses and indications are compared to DDD values.2 However, the relationship between prescribing and reported use from pharmacy information systems is further complicated by the amount of drug discarded in the process of preparing individualised doses from standard sized vials.3,8
This study explored the levels of agreement between adult DDD and feasible paediatric estimates of daily antibiotic use (numerators) in the context of a paediatric hospital that does not have access to individual patient-level data.
METHODS
Setting
This retrospective study was conducted in a 170-bed university affiliated tertiary paediatric hospital in New South Wales, Australia. The hospital is adjoined by two public hospitals for general adult and specialist women’s and newborn care. A range of services are shared across the campus including operating theatres, radiology and pharmacy. The hospital’s level six paediatric intensive care unit (PICU) accepts complex surgical and oncology patients from birth to 18 years, including preterm neonates transferred from the neonatal intensive care unit (NICU) at the adjoining hospital.
Nursing staff order routinely prescribed antimicrobials that have been approved as “imprest” items from a pharmacy warehouse shared between the adult and paediatric hospitals. Pharmacy warehouse staff distribute imprest items to individual wards with limited or no direct contact with pharmacists; non-imprest antimicrobials are dispensed as whole vials to individual patients by pharmacists in the hospital dispensary. Pharmacists review and endorse both imprest and non-imprest medications prescribed by doctors on paper-based medication charts but do not collect data on the patients or medications reviewed.
Due to these ordering methods antimicrobial use is not consistently linked to individual patients. All injectable medications, other than those associated with high cost or special handling requirements, are prepared by nurses on the ward from whole vials. State-wide infection control and medication handling policies mandate the use of single dose vials over multi-dose products and require nurses to discard any unused portions of injectable medicine.9
Data collection and analysis
Antimicrobial and patient demographic data
Records of antimicrobial supply to PICU inpatients from 1 January 2010 to 31 May 2016 were extracted from the hospital pharmacy information system (iPharmacy, CSC, Sydney Australia). In keeping with the National Antimicrobial Utilisation and Surveillance Program methods used for adult hospitals in Australia, the data combined records of imprest distribution from the pharmacy warehouse and individual inpatient dispensing by pharmacists.4 Discharge and outpatient dispensing associated with the PICU cost centre code were excluded. WHO Collaboration Centre for Drug Statistics Methodology Anatomical Therapeutic Chemical (ATC) classification system for antimicrobials category J01, and J04 injectables were extracted.10
Tobramycin and colistin for injection and inhalation could not be differentiated consistently throughout the study period and were excluded from further analysis. Injectable erythromycin was also excluded because it is more commonly prescribed for gastric motility in our institution. Gentamicin was excluded due to a transition from 8 hourly to once daily gentamicin for neonates and oncology patients during the study period, sporadic supply of two vial sizes to the PICU via imprest.
Data entry errors were corrected after confirmation from pharmacy and warehouse managers; records of unused items returned to stock after initial supply were subtracted from the original month of supply. Antibiotic use for each agent was reported as monthly vial counts according to vial size in grams. Date of birth and occupied bed-day (OBD) data were obtained from the hospital performance unit. Patient age (in months) was calculated for each patient at each day of their PICU admission and used to create a database of monthly age-specific PICU occupied bed-days.
Measures of antibiotic use
Three paediatric measures of daily antibiotic consumption were derived from the dosage and frequency recommendations published in the national paediatric medication reference text and the New South Wales Neonatal Medicines Formulary.11,12 Where there were no local or national recommendations these were obtained from Lexi-Comp® via UpToDate and the British National Formulary for Children.13,14 For consistency across measures reference dosages and frequencies were defined. Where possible, the reference dosage in milligram per kilogram and reference frequency were roughly equivalent to the adult values assigned by WHO. For example, DDD assignments for J01CR beta-lactams with beta-lactamase inhibitors (piperacillin-tazobactam and ticarcillin-clavulanate) were an average of two commonly prescribed dosage schedules, therefore, the same approach was taken. Where the DDD assignment reflected maintenance or severe infection, the same principle was applied to the reference dosage and frequency. Patient gender and actual weight was not available, therefore, the median weight for age (in months) was obtained from the United States Centers for Disease Control and Prevention weight-for-age percentile reference ranges for girls.15
Paediatric measure 1: Estimated daily use of vials
The estimated daily use of vials was a fixed value equal to the reference frequency children. A single vial was assigned to each dosage administration within 24-hours without modification for patient weight, age, dosage delivered, or vial size supplied. For example, the estimated use of vials for vancomycin was fixed at four vials per day despite the fact that some patients would require more than a single vial for each dosage administration due to age, weight or the prescribed dose.
Paediatric measure 2: Age-adjusted daily use of vials
Age-specific dosage and frequency, potential residual antibiotic and age-specific occupancy in PICU (age-specific occupied bed days) were incorporated into the age-adjusted daily use of vials. First, individual dosages were calculated for each antibiotic for ages 3 months to 18 years (reference dosage in mg/kg x 50th percentile weight-for-age, up to the maximum reference dosage). The number of whole vials required to administer the calculated dosage was assigned according to the vial sizes supplied to PICU; dosages were allowed to be rounded down to the nearest whole number of vials where the dosage delivered would remain within 5% of the calculated dosage. Average daily vial requirements were obtained by multiplying the number of whole vials required per dosage administration by the reference frequency. Average daily vial requirements for neonates broadly accounted for gestational age by taking the lowest or most commonly used frequency in neonates. Unless otherwise stated, gestational and postnatal age-adjustment was applied uniformly to all patients under 3 months old to account for possible preterm birth.
The age-adjusted daily use of vials was calculated for each month of the study period, by applying the proportion of age-specific PICU occupied bed days to the corresponding average daily vial requirements for each age. i.e., Age-adjusted daily use of vials= ∑ (Average daily vial requirement for age × Proportion of occupied bed-days for age).
Neonatal dosage adjustments were not performed for antibiotics that were deemed rare or unsuitable for neonatal use. The proportion of age-specific occupied bed-days was recalculated accordingly.
Paediatric measure 3: Recommended daily dose (RDD)
The median PICU admission age each month was used to select the age-specific reference dosage (mg/kg), reference frequency and 50th percentile weight used to calculate the monthly RDD unit of measure i.e., RDD = (Reference dosage in milligram per kilogram × reference frequency) × 50th percentile weight for age.
PICU antibiotic use
Monthly use of each agent in PICU was reported as the number of WHO ATC DDDs ([vial count × vial size]/ ATC DDD 2016); the number of estimated daily use of vials (vial count/Estimated daily use of vials); the number of age-adjusted daily vials (vial count /Age-adjusted daily use of vials and the number of RDDs ([vial count × vial size]/ RDD). Notably, PICU antibiotic use in measured in RDDs and age-adjusted daily use of vials were obtained by dividing monthly use by the age-adjusted daily use of vials or RDD unit of measure for the month in question.
Ethics
Ethics approval was granted by the hospital Human Research Ethics Committee (LNR/16/SCHN/445) and ratified by the University of Technology Sydney.
Statistical analysis
Data was extracted to a Microsoft Excel 2016 database (Microsoft Corporation, Redmond, WA, USA) for initial calculations. Statistical analysis was performed in R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Descriptive statistics were used to report the monthly age-adjusted estimated daily use of vials measure that resulted from the PICU patient population throughout the study period. Agreement between the DDD and the estimated daily use of vials, the DDD and the age-adjusted daily use of vials, and the DDD and the RDD was assessed visually via Bland-Altman plots for each antibiotic with at least 10 months of use in the PICU (10 observations).
Differences in estimated monthly use between DDD and each of the paediatric use measures were plotted against the Averages of the two measures, i.e. y = Differences = use in DDD – use in paediatric measure, x=Averages = (use in DDD + use in paediatric measure)/2. Shapiro-Wilk tests and visual inspection of Bland-Altman and quantile-quantile plots confirmed whether the calculated differences were normally distributed. Where the assumption of normality was not met linear regression was used to describe the mean difference as a function of Averages. As described by Bland and Altman, the mean differences are obtained from a fitted regression model (model 1), B0 + B1Averages = Differences. The limits of agreement are then derived from a second linear regression model, C0 + C1Averages = Residuals, where the residuals are the absolute residuals from model 1. Statistical significance was determined by the p-value of the coefficients of the Averages, Β1 and C1. The limits of agreement were calculated as ± 2.46 (C0 + C1Averages) of the mean difference (B0 + B1Averages).16 Where distributions varied between antibiotics the most common distribution determined the method of analysis, and a single approach was applied to antibiotics. All tests were two-tailed, and p-values <0.05 were considered statistically significant.
RESULTS
Monthly OBD in the PICU ranged from 228 in January 2010 to 510 in July 2013 (mean=388, SD=64). Median PICU admission age was 16 months (IQR 3 months – 6 years 6 months). Of the 30 different injectable antibiotics supplied to the PICU throughout the study period, 60% (18/30) were supplied in one vial size. Cefotaxime was the only antibiotic supplied in more than two sizes. A reference dosage and frequency was assigned for all the included antibiotics.
For 12 antibiotics that were limited to a single vial size, the estimated daily use of vials was equal to adult DDD in terms of the reported grams of use, and the number of vials required (Online appendix: Table 1). These were; azithromycin, cefalotin, cefazolin, imipenem, lincomycin, metronidazole, moxifloxacin, piperacillin-tazobactam, rifampicin, teicoplanin, ticarcillin-clavulanate and tigecycline. Whilst amikacin, aztreonam, cefoxitin, linezolid, daptomycin, trimethoprim-sulfamethoxazole were also supplied in a single size the estimated daily use of vials was not equal to DDD (Online appendix: Table 1). For eight antibiotics the measure accounted for the average daily vial requirements in all patients after the neonatal period, despite 2-fold or greater variation if reported as DDD (ampicillin, cefepime or flucloxacillin = 1.0–2.0 DDD, meropenem and ceftazidime = 0.75–1.5 DDD, clindamycin = 0.5–1.0 DDD, benzylpenicillin ~0.67–1.33 DDD, cefotaxime = 0.5–2.0 DDD).
Table 1. Characteristics of 400 elderly patients
| Variables | Median | (P25, P75, IQR) |
|---|---|---|
| Age | 70.5 | (67, 75, 8) |
| Female – n (%) | 264 | (66) |
| Number of diseases | 3 | (2, 4, 2) |
| Number of medications | 11 | (5, 25, 20) |
| Number of prescriptions | 3 | (2, 6, 4) |
IQR: interquartile range; P25: percentile 25%; P75: percentile 75%.
Almost half of the included agents (14/30) had an estimated daily use in vials that accounted for average daily vial requirements for all patients, regardless of age-occupied bed days assigned to neonatal and older children (Online appendix: Table 1). Neonatal daily vial requirements varied from the reference frequency for children (i.e., estimated daily use of vials) for nine agents, (ampicillin, benzylpenicillin, cefazolin, cefotaxime, ceftazidime, flucloxacillin, metronidazole, ticarcillin-clavulanic acid and vancomycin). Calculated average daily vial requirements for age identified antibiotics that required changes to the estimated daily use of vials to account for weight or age in children (cefoxitin, ceftriaxone, aztreonam, trimethoprim-sulfamethoxazole, amikacin, ciprofloxacin, vancomycin, linezolid).
The age-adjusted daily use of vials for vancomycin (Figure 1) varied more than any other agent due to differing average daily vial requirements in both neonates and children (range 3.3–5.0 vials), followed by cefoxitin (range 3.3–4.6 vials) and trimethoprim/sulfamethoxazole (range 2.3–3.2 vials).
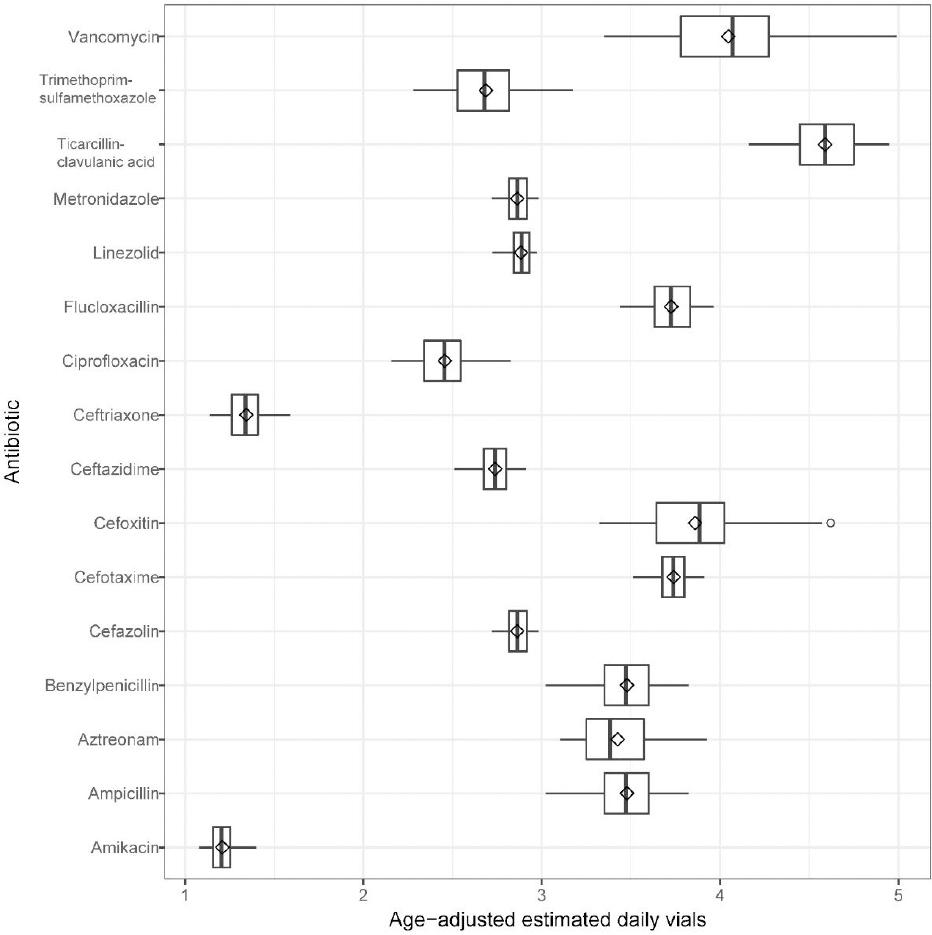
Figure 1. Age-adjusted estimated daily vials unit of measure generated from age-specific occupied bed-days in the Paediatric Intensive Care Unit. (values reported in Online appendix: Table 1).
Agreement between reported use in DDD and the paediatric units of measure was completed for the 20 antibiotics that were supplied to the PICU during 10 or more months of the 77 month study period. Shapiro-Wilk tests of the differences confirmed normal distributions for only two antibiotics and thus regression methods were used to determine the mean difference and limits of agreement. Bland-Altman plots of PICU use in DDD and estimated daily use of vials showed perfect agreement for azithromycin, cefalotin, cefazolin, lincomycin, metronidazole, piperacillin-tazobactam and ticarcillin-clavulanic acid (7/20).
The relationship between the differences between DDDs and estimated daily use of vials used in PICU versus their averages was perfectly linear for amikacin, trimethoprim–sulfamethoxazole and linezolid, as these were all limited to a single vial size there was no deviation from the regression lines (Figure 2). The mean difference for vancomycin in DDD and estimated daily use of vials was not statistically significant (p=0.059), with only three deviations attributed to months during which a small number of larger sized vancomycin vials were supplied.
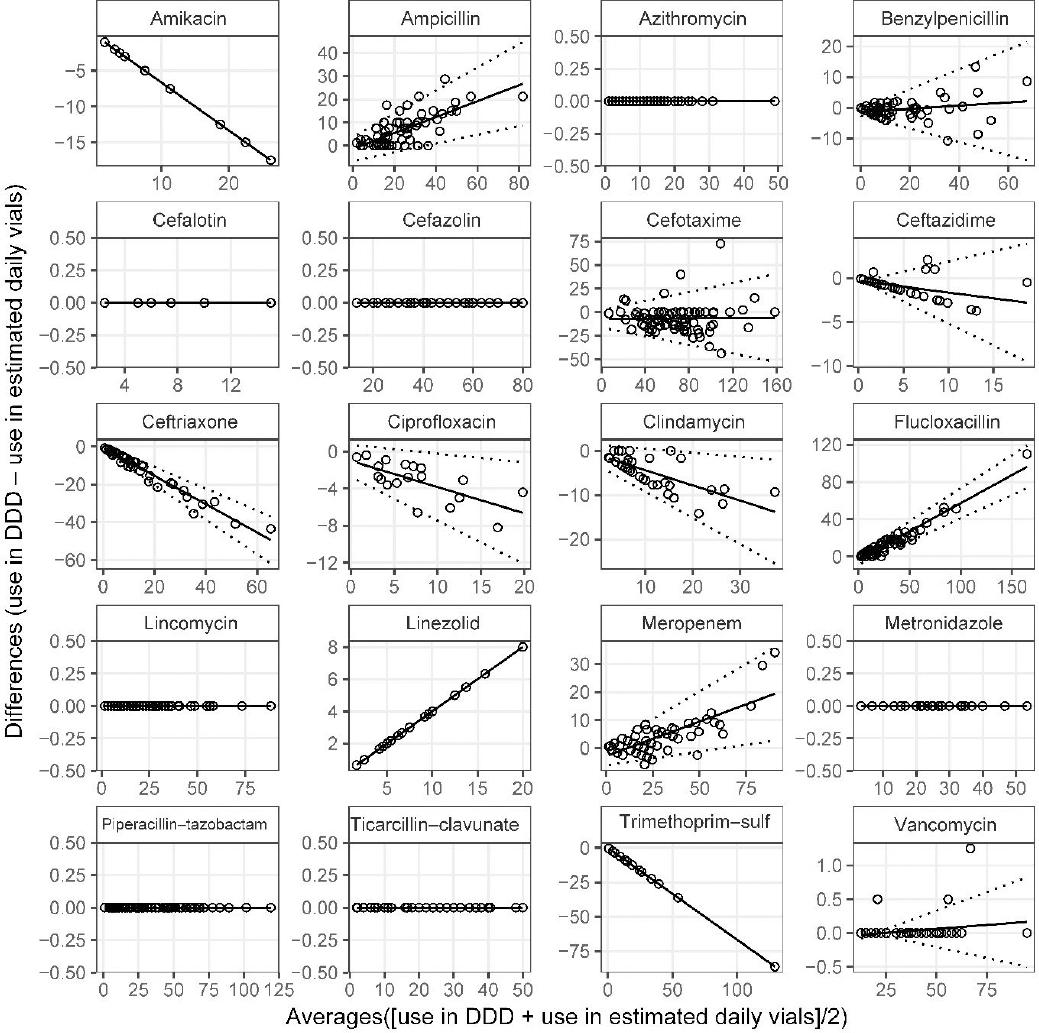
Figure 2. Bland-Altman plots of World Health Organization defined daily doses and Estimated daily use of vialsMean difference (solid line) and limits of agreement (broken lines) obtained from linear regression (Online appendix Table 2, DDD vs Estimated daily use of vials).
Table 2. Top five PIMs by criteria (2016-2017)
| Rank | Overall | Rank | Winit-Watjana | ||||
|---|---|---|---|---|---|---|---|
| PIMs | N | (%) | PIMs | n | (%) | ||
| 1 | Orphenadrine | 255 | (15.9) | 1 | Orphenadrine | 255 | (22.7) |
| 2 | NSAIDs a | 231 | (14.4) | 2 | NSAIDs a, e | 231 | (20.5) |
| 3 | ACEI b | 208 | (13.0) | 3 | ACEI b | 208 | (18.5) |
| 4 | Dimenhydrinate | 155 | (9.7) | 4 | Benzodiazepine c | 112 | (10.0) |
| 5 | Benzodiazepine | 112 | (7.0) | 5 | Flunarizine | 101 | (9.0) |
| 6 | Others | 642 | (40.0) | 6 | Others | 218 | (19.4) |
| Total | 1603 | (100.0) | Total | 1125 | (100.0) | ||
| Rank | 2015 Beers | Rank | STOPP version 2 | ||||
| PIMs | n | (%) | PIMs | n | (%) | ||
| 1 | Orphenadrine | 255 | (32.7) | 1 | Benzodiazepine | 112 | (22.2) |
| 2 | Dimenhydrinate | 155 | (19.8) | 2 | 1st-generation antihistamine | 108 | (21.4) |
| 3 | Benzodiazepine | 112 | (14.3) | 3 | Opioid | 96 | (19.0) |
| 4 | 1st-generation antihistamine d | 108 | (13.8) | 4 | NSAIDs e | 79 | (15.6) |
| 5 | Omeprazole | 78 | (10.0) | 5 | Omeprazole | 78 | (15.4) |
| 6 | Others | 73 | (9.3) | 6 | Others | 32 | (6.3) |
| Total | 781 | (100.0) | Total | 496 | (100.0) | ||
anonsteroidal anti-inflammatory drugs
bangiotensin-converting enzyme inhibitors
cexamples of benzodiazepine: lorazepam and alprazolam
dexamples of 1st-generation antihistamine: brompheniramine, chlorpheniramine and hydroxyzine
eNumbers of NSAIDS differed depending on criteria
As expected, agreement varied for antibiotics that were supplied in multiple vial sizes. Flucloxacillin plots exhibited the narrowest limits of agreement, and the most prominent slope (Figure 2). The steep incline suggested that higher usage months measured in DDD may vastly overestimate days of use compared to estimated daily use of vials. Bland-Altman plots for ampicillin produced wider limits of agreement as various vial sizes were more consistently used, including lower usage months. One in every four months of cefotaxime use were in perfect agreement (19/76, difference=0) without a statistically significant change in the mean difference in relation to the averages (p=0.922). Differences between cefotaxime use in DDD and estimated daily use of vials were both negative and positive, suggesting DDD measures could potentially under- and over-report use. The largest negative difference and most extreme positive outlier occurred during periods of high cefotaxime use, the former when large quantities of predominantly small vials were supplied (difference= -43.73, 67% vials 0.5g), the latter when larger vial sizes were supplied difference = +72.5, 100% of vials 2g) (Figure 2).
After adjustment for daily vial requirements and age-specific OBD, metronidazole, cefazolin and ticarcillin–clavulanate were no longer in perfect agreement (Figure 3). However, the impact on the differences remained small (Online appendix: Table 2). Differences between use in DDD and age-adjusted daily use of vials for ampicillin and flucloxacillin remained statistically significant, and positive in relation to the averages. Despite changes in the appearance of the cefotaxime and vancomycin plots, the mean differences were not statistically significant (Online appendix: Table 2); the limits of agreement were, however, narrower and wider respectively as expected. The magnitude of the mean difference in relation to the averages changed significantly after age adjustment to benzylpenicillin and ciprofloxacin as demonstrated by the changes to the slopes (Figure 2 and Figure 3).
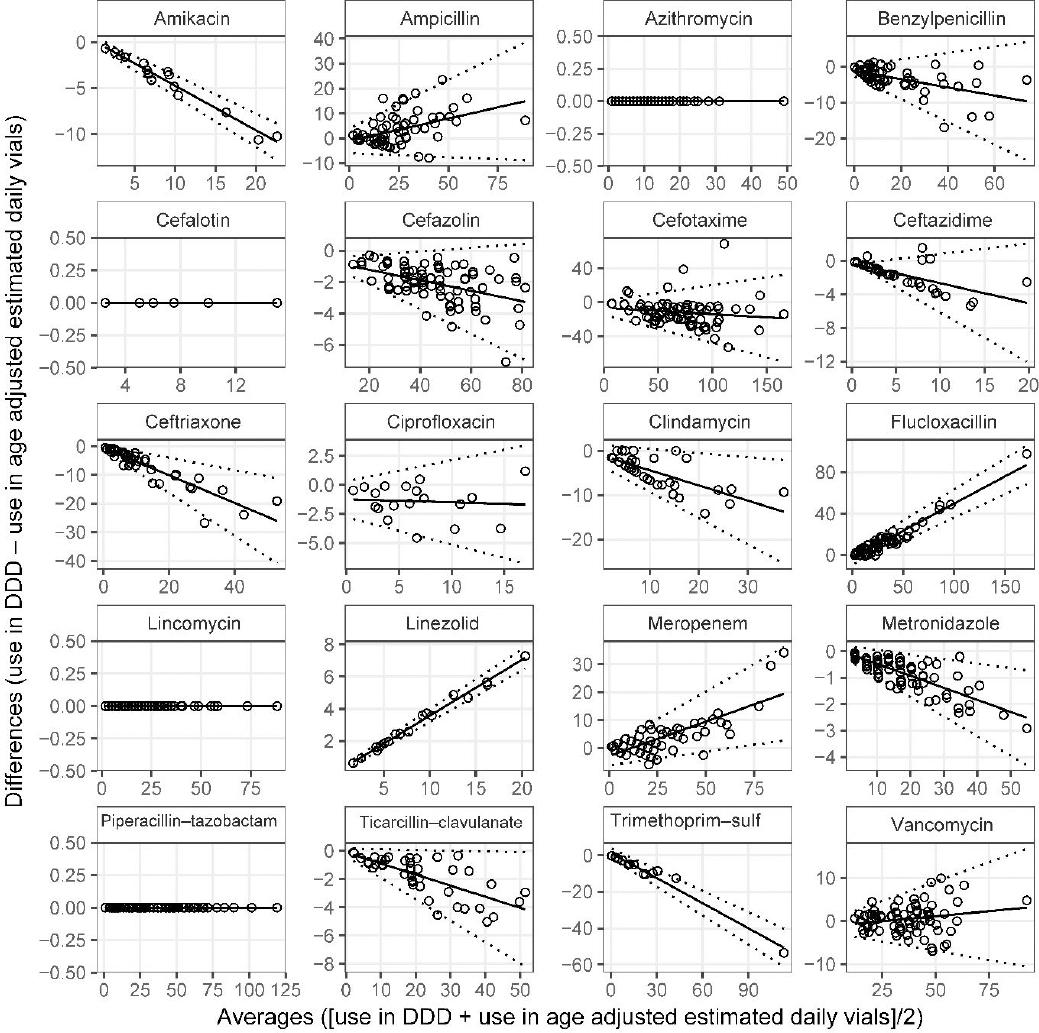
Figure 3. Bland-Altman plots of World Health Organization defined daily doses and Age-adjusted estimated daily use of vialsMean difference (solid line) and limits of agreement (broken lines) obtained from linear regression (Online appendix Table 2, DDD vs Age-adjusted estimated daily vials).
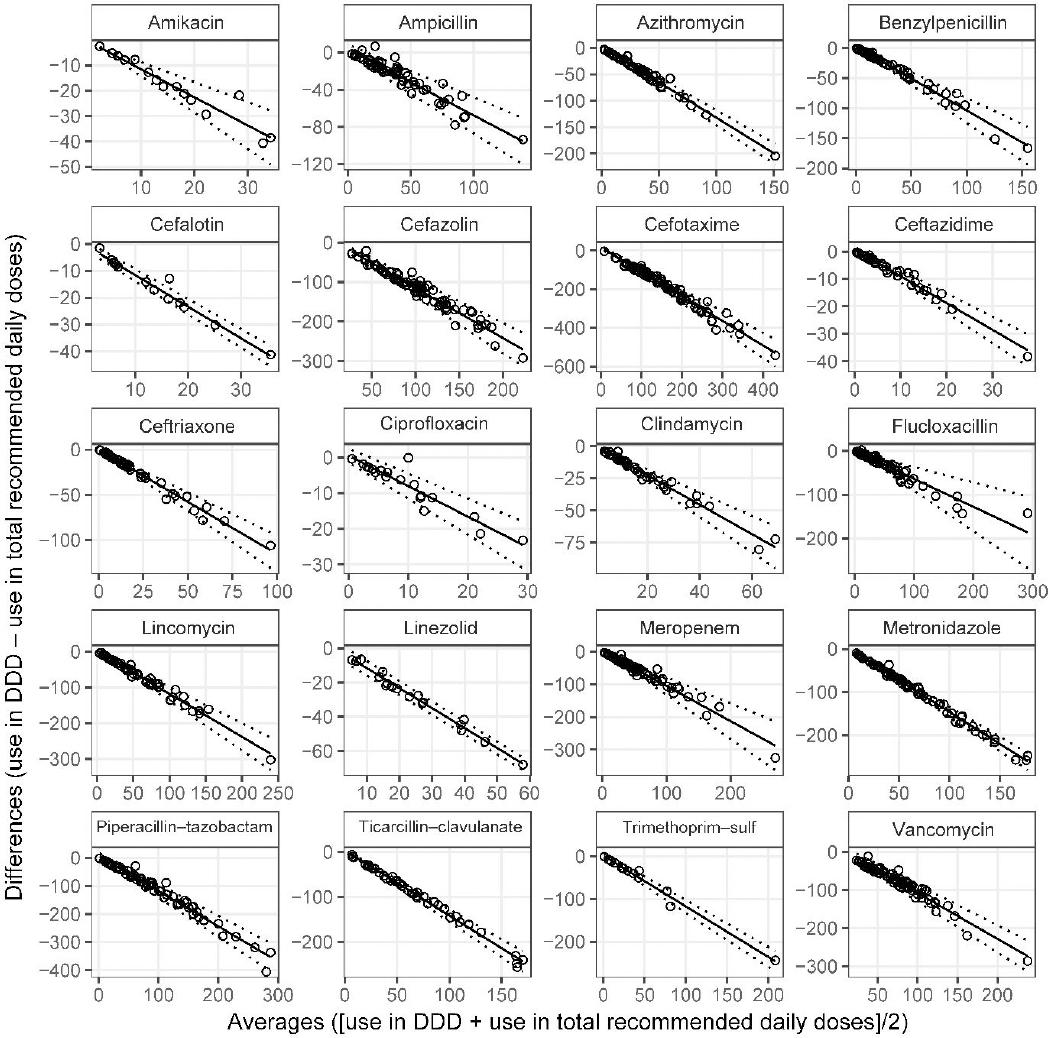
Agreement between DDD and total recommended daily doses was poor. Visual inspection of Bland-Altman plots and linear regression showed obvious discrepancy between use in DDD and RDD, the relationship between the differences and the averages of the two measures was statistically significant and inversely proportional (Figure 4). Differences between DDD and RDD increased dramatically with higher average use; negative differences indicated that the estimated days of antibiotic use measured in RDD far exceeded that which was reported in DDD.
DISCUSSION
Despite its exploratory nature, this study offers some insight into a range of patient and organisational factors such as pharmacy distribution systems, single-vial policies and external factors such as drug shortages that influence the approach to antimicrobial surveillance in children’s hospitals. Extracted records of use together with medication reference texts identified a range of antibiotics that are likely to be reliably reported in children and teenagers in DDD without any adjustment, and with only minor adjustment in neonates. This list of agents includes 10 that are restricted or highly restricted agents in our hospital. Also included is injectable metronidazole, which, while unrestricted, is a potential target for antimicrobial stewardship activities that promote IV to oral switch or reduce therapeutic duplication. Approximately half of the antibiotics used in PICU required estimated daily use of vials measure to be adjusted for age and weight and only one antibiotic, vancomycin, required adjustment for both neonates and children.
Bland-Altman plots of antibiotic use measured agreement between each of the paediatric antibiotic use measures and DDD, illustrating how vial size, age and waste may impact drug usage reports from pharmacy supply records. Compared to DDD, the vial-based units of measure appeared more robust against cefotaxime formulary change and drug shortage by focusing on the dosage frequency, rather than the actual dose. In contrast, agreement between PICU use measured in DDD and RDD suggested RDD would report considerably higher estimates of monthly use, even where all other measures were equal or similar.
To the best of the author’s knowledge, previous weight-adjusted methods using pharmacy data have largely focused on the dosage prescribed reporting use in RDD or proportions of DDD rather than vial requirements.3,8,17,18 For example, Liem et al. suggest that “neonatal DDD” for ampicillin could be one tenth of the adult DDD values.3 Applying the single vial policies in the study hospital would result in a reported daily use of 1g (2 vials) to 4 g (4 vials) of ampicillin depending on gestational and postnatal age. Others have argued against weight-based adjustments due to the broad range of paediatric doses and the wide range of indications, choosing to use DDD for benchmarking and trend analysis.19 Whilst DDD generally appeared to be closer to the minimum quantity reported for a single day of use in this setting, these conclusions may not be generalisable to hospitals without single vial policies, and different vial sizes in use. These variations are likely to limit the capacity for benchmarking between hospitals and comparisons with published surveillance reports internationally.
This study has a number of other limitations. The measures developed in this study were modelled similar to DDD and share some of the same limitations, including that they might be based on recommended dosages that do not accurately reflect the most common dosage regimens actually used in hospitals. Prescribers may choose alternate regimens within the medication reference range for convenience (i.e., to limit the number of daily dosages), severity of infection or presence of comorbidities (e.g., renal impairment). However, these concerns are not limited to vial-based measures or children and are likely to be present in adult hospitals that use DDD. Vial-based measures may not identify a shift to higher dosages in milligram per kilogram that do not change the daily vial requirements and may overestimate use when multiple smaller vials are used to deliver a dose that could have been administered with a single vial. Furthermore, the age adjustments applied in our study are estimates. Complete records of gestational age were not available and patients under 3 months old were assumed to be neonates. In addition to possibly over-estimating the adjustments required for neonates, this also meant detailed adjustments could not be made for postnatal vial requirements. For children and teenagers, average daily vial requirements were extrapolated from standard paediatric weight-for-age reference ranges and not actual patient weight. Due to the preliminary nature of this work we did not perform any a priori sample size calculations, nor did we assess agreement as a function of time. While these are limitations of this study, they may be overcome in future studies; gestational and postnatal age are collected by Australian PICU’s and measured weights are now available for electronic extraction in the study hospital. Age adjustments may also be influenced by the ordering process if antibiotics were supplied and administered in separate months. Finally, the measures were not compared to actual days of use. Such validation would require a prospective observational study or access to electronic medication administration or prescribing data, which were neither feasible nor available. However, this work is an important initial exploration in an area in which there remains an unmet need and highlights the need to include detailed information on the local setting when reporting on antimicrobial use. Namely, pharmacy drug distribution systems and relevant infection control or medication handling policies. In addition, the principles applied in this study may be utilised for other injectable medications in hospitals with similarly limited pharmacy services, particularly those with a narrow dosage range.
Further research is needed to assess whether agreement between estimated vial-based measures or selected antibiotic DDD and actual use are acceptable for local surveillance, national benchmarking programs and/or epidemiological studies. Initial studies should investigate drug distribution systems, presence of single vial policies, hospital formularies and medication dosage guidelines for similarities. Broad-spectrum agents associated with hospital acquired and resistant infections including, but not limited to carbapenems, vancomycin, linezolid and daptomycin should be prioritised. Consensus based methods may be required to reconcile discrepancies between prescribed doses and reference ranges used to define paediatric measures.
CONCLUSIONS
Paediatric antibiotic use reports generated from pharmacy information systems may not reflect actual administration because of the influence of variable vial size, patient age, pharmacy drug distribution systems and local medication handling and infection control policies. These factors should be assessed before inter-hospital comparisons are made. Agreement between the DDD and estimated daily vials and age-adjusted daily vials were superior to total recommended daily doses and unchanged by drug shortages in a PICU with a single vial policy. In this setting, a considerable number of antibiotics targeted by AMS programs may be reported in DDD when used for children and teenagers.