INTRODUCTION
Fluoroquinolones (e.g., ciprofloxacin, levofloxacin, moxifloxacin, ofloxacin, and gemifloxacin) are the third most commonly prescribed class of antibiotics in the United States in outpatient settings and the most commonly prescribed class of antibiotics in hospitals.1,2 Resistance to fluoroquinolones among an important group of Gram-negative bacterial pathogens (e.g., Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumonia) has increased significantly, highlighting the need for providers to scrutinize the use of fluoroquinolones in patients infected with these pathogens.3,4 Of major concern is that fluoroquinolones are associated with a range of disabling and potentially irreversible adverse reactions, including reactions involving the musculoskeletal and peripheral nervous systems (tendonitis and tendon rupture, muscle and joint pain, peripheral neuropathy) and the central nervous system (psychosis, anxiety, insomnia, depression, suicidal thoughts, hallucinations); significant decreases in blood sugar and attendant risk for coma; and ruptures or tears in the aorta.5
In July 2016, the U.S. Food and Drug Administration (FDA) updated the boxed warning for fluoroquinolones to indicate that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis in those with chronic obstructive pulmonary disease (ABECB-COPD), and uncomplicated urinary tract infections (uUTIs).6 Importantly, a recent study showed that 5% of all fluoroquinolone prescriptions in the United States in 2014 were given for conditions for which no antibiotics are indicated, and 20% were given for conditions for which fluoroquinolones are not recommended first-line therapy, including 6.3 million for sinusitis and uUTIs and 1.6 million for viral respiratory tract infections and bronchitis.7 Any reduction in inappropriate prescribing of fluoroquinolones will reduce preventable harm from this class, with a concomitant reduction in associated health care costs such as the cost of drugs and their administration, costs of treatment of side effects, and costs of future antibiotic resistance.8
Many clinicians do not believe they prescribe antibiotics inappropriately, yet when faced with feedback comparing their own prescribing patterns with those of peers, they will modify their personal prescribing patterns.9,10 Much of the power of feedback lies in showing clinicians that their own behaviors are inconsistent with a desirable target or with what their peers are doing.11,12 To date, such feedback, delivered in verbal and written formats, has demonstrated moderate effectiveness in changing antibiotic prescribing behaviors.13 14-15 Questions remain, however, about the degree of effectiveness associated with different feedback formats and how best to optimize the effect of feedback interventions designed to change prescribing behaviors.12,16
The FDA Safe Use Initiative funds research to develop innovative methods (including education, novel messaging strategies, and mobile technologies) to facilitate research in the area of safe medication use and was the impetus for this study, which is a collaboration between the FDA and Medscape, an online provider of medical information and continuing education for clinicians.17Medscape's parent company, WebMD, proposed this study in response to the Food and Drug Administration Broad Agency Announcement for the Advanced Research and Development of Regulatory Science (FDABAA-15-00121) and was awarded funding for research that the FDA anticipates “will serve to advance scientific knowledge to accomplish its mission to protect and promote the health of our nation.” Interventions for this study were developed in collaboration with the FDA's Center for Drug Evaluation and Research. In this study, we investigate the effects of feedback and education about fluoroquinolone prescribing on high-volume prescribers of fluoroquinolones when delivered to them as 1) targeted short-form messages and 2) a continuing medical education (CME)-certified activity, individually and in combination. A targeted short-form message can be thought of as short form content targeted to a specific audience (in this case high prescribers) and delivered via a concise messaging format (in this case emails, web alerts, and mobile alerts). Short form content is typically 400-600 words, can be read in as little as two minutes, and doesn't require much critical thinking.18Primary outcomes were the volume of new prescriptions written and the number of providers reducing their prescribing (Prescriber Reducers). Subgroup analyses measured new prescription volume by tactics (targeted short-form message alone, targeted short-form message with CME, or CME alone), message (targeted short-form message format A, B, or C), and indication (ABS, ABECB-COPD, and uUTI), and Prescriber Reducers by specialty subgroup (PCPs, urologists, all other physicians, and nurse practitioners/physician assistants). Email open rates for targeted short-form messages were also measured to assess degree of engagement with three different messages.
METHODS
Study Design
We conducted an ex post factomatched control study between October 2016 and July 2017 using Medscape's online capabilities to drive targeted short-form messages and education to a large number of high-volume fluoroquinolone prescribers among Medscape's online clinician membership. We devised a three-phase approach to 1) define the target audience for messaging and education; 2) develop and implement targeted short-form messages and educational (CME and non-CME) content; and 3) evaluate the impact of targeted short-form messages and education.
Phase 1: Define the Target Audience
A population of 320,478 Medscape prescriber members, representing all prescribing deciles (1 to 10), prescribed approximately 14.5 million prescriptions for fluoroquinolones during a pre-intervention period from September 2014 to August 2015. From this population, we identified a target audience of 28,004 high-volume fluoroquinolone prescribers (deciles 6 to 10); this target audience, which included physicians, nurse practitioners, and physician assistants, wrote 7.3 million fluoroquinolone prescriptions over the 12-month period at an average of 260 prescriptions per member.
The target audience was identified by a process in which validated US-based Medscape members with a history of recent activity on Medscape and an email address registered with Medscape were matched to third-party data sources from IQVIA (formerly Quintiles and IMS Health). IQVIA is a global provider of data, technology, and analytics for the healthcare industry. IQVIA provided de-identified prescriber-level data for physicians, nurse practitioners, and physician assistants based on pre-intervention, 12-month (May 2015 to April 2016) fluoroquinolone prescribing activity using unique identifiers (Medical Education number or National Provider Identifier number and other data points such as specialty, profession, name, and geographic location). IQVIA analysis of provider total prescribing volume for the 12-month period included fluoroquinolone medications that are currently on the market and indicated for systemic administration. We also identified fluoroquinolone prescriber physician specialties using Medscape's membership records and determined the total prescribing volume by specialty using the IQVIA VOPEX (Vector One: Prescriber Extract) service offering, which tracks HCP medical claims data and prescription volumes by product.
Phase 2 A: Development and Implementation of Targeted Short-Form Messages and Non-CME Education
A series of targeted short-form messages was developed in collaboration with the FDA's Center for Drug Evaluation and Research. We tested thirteen subject lines, each with an average of 1,666 emails (range: 1,206 - 2,397). Engagement with each targeted short-form message was evaluated by unique (de-duplicated) email open rates. The three targeted short-form messages with the highest unique open rates were used in the full intervention. Targeted short-form messages included a link to an online (medscape.com) educational (non-CME) resources center that highlights key messages and clinical data on fluoroquinolone prescribing.
Each of the three targeted short-form message formats had a unique subject line and content:
Message A: Subject line: “Your fluoroquinolone usage data.” Content: A brief message on the prescriber's current fluoroquinolone prescription volume as derived from IQVIA data compared with the national average for their specialty
Message B: Subject line: “Your fluoroquinolone prescribing volume exceeds the average.” Content: A brief message on the prescriber's current fluoroquinolone prescription volume compared with the national average for their specialty, combined with clinical context (i.e., FDA drug safety review findings and label update with advisory on appropriate fluoroquinolone prescribing)
Message C: Subject line: “Serious side effects prompt FDA to change fluoroquinolone labeling.” Content: Clinical context only
Phase 2 B: Development and Implementation of CME
An online CME activity (Improving the Safe Use of Fluoroquinolone Antibiotics, https://www.medscape.org/viewarticle/870313) was jointly developed by the FDA. This CME-certified activity (0.75 AMA PRA Category 1 Credit™) was co-authored by an FDA therapeutic expert and Medscape and provided a review of scientific advances in fluoroquinolone prescribing. Content was driven by an educational needs assessment which indicated HCP knowledge gaps regarding efficacy and safety data for fluoroquinolones and the appropriate role of this drug class in the context of treating patients for ABS, ABECB-COPD, and uUTI. Custom recruitment on medscape.org included targeted promotion to the entire 28,004-provider target list.
Phase 3: Evaluation of Intervention Impact
The outcome measures for evaluating the intervention impact were:
Test versus control difference for new prescription volume of fluoroquinolones
Test versus control difference for percentage (%) of HCPs reducing their prescribing of fluoroquinolones (Prescriber Reducers). In this study, Prescriber Reducers were defined as healthcare providers who reduced their average monthly prescribing of fluoroquinolones volume by any amount from the pretest period to the test/posttest period.
Subgroup analyses were performed to determine test versus control differences for:
New prescription volume by tactics (Targeted short-form message alone, Targeted short-form message with CME, or CME alone)
New prescription volume by message (Targeted short-form message format A, B, or C)
Prescriber Reducers by specialty subgroup (PCPs, urologists, all other physicians, and nurse practitioners / physician assistants)
New prescription volume by indication (ABS, ABECB-COPD, and uUTI)
Prescriber engagement with targeted short-form messages were evaluated by email opens.
Sample Size
It was estimated that a minimum of 10,000 members of the target audience would be engaged in one of the three tactics of the intervention: targeted short-form message alone, targeted short-form message with CME, or CME alone. An engagement is defined as opening an email, viewing an alert, or participating in CME The 10,000 member minimum engagement was estimated based on the size of the targeted audience, anticipated open rates for emails, anticipated views of web and mobile alerts, anticipated number of participants in the CME activity, and Medscape's experience communicating with health care provider audiences.
Power analysis was also done to verify that with such a sample size there will be at least 80% power to detect a significant program effect, if it exists, for Message A only, Message B only, and Message C only segments. The sample size for CME content only, Message A with CME, Message B with CME, and Message C with CME was anticipated to be considerably lower and was thus not included in the power analysis.
Of the targeted high-prescribing HCPs, a participant test group of 11,774 HCPs was matched to the IQVIA prescriber universe to identify non-participant control HCPs based on the following criteria:
Oral fluoroquinolone new prescription volume in the pretest period
Market trend in oral fluoroquinolone new prescription volume in the pretest period
Profession (physician, NP, PA)
IQVIA physician specialty
HCP geography
Overall writing decile
Intervention
Test HCPs were randomized into three segments to measure the effectiveness of three unique targeted short-form message formats (Message A, B, or C). Targeted short-form messages were delivered as emails and Medscape website and mobile alerts. A fourth segment of high-volume fluoroquinolone prescribers did not receive any targeted short-form messages but participated in the CME activity only.
Targeted short-form messages
Email. Targeted short-form message emails were sent from Medscape to test HCPs in four waves over the six-month period from late October 2016 to late April 2017 (Figure 1); each of the two initial email waves was followed by another email wave that targeted only test HCPs who did not open the initial email (for a total of four waves).
Website and mobile alerts. Targeted short-form message alerts appeared on a target provider's web browser or mobile device when visiting medscape.com. (Medscape users log on to the system and are thus identifiable.) High volume fluoroquinolone prescribers (deciles 6 to 10) were presented with a personalized message concerning fluoroquinolone prescribing. Alerts were displayed during an HCP's session in two different ways: as full messages that are displayed in the context of relevant content, and as linked headlines. Website and mobile alerts were delivered in two waves over the period from late January 2017 to late February 2017 (Figure 1).
Continuing medical education
Recognizing that urology and primary care are specialties commonly associated with fluoroquinolone prescribing, Medscape members who were high-volume fluoroquinolone prescribers were identified and grouped according to specialty (i.e., urologists, primary care physicians [PCPs], all other physicians, and nurse practitioners/physician assistants) and were recruited to an online, interactive, text-based, CME-certified activity on safe use of fluoroquinolones based on an analysis of prescriber-level data. Recruitment was conducted via unique messages strategically placed on targeted medical specialty homepages. The messages were placed in areas of the specialty homepages focused on educational content most relevant to treatment of ABS, chronic obstructive pulmonary disease (COPD), and uUTI in order to drive the most relevant HCPs to the available education. Recruitment to the activity occurred at activity launch and continued for the first 3 months. The activity was hosted on medscape.org from January 2017 to January 2018 and was available exclusively to the target audience during the first 6 months of its certification (Figure 1).
Data Analysis
The test period was defined as November 2016 to April 2017; the posttest (measurement) period was defined as November 2016 to July 2017 (Figure 1). Some HCPs who were late October 2016 participants were included as November 2016 participants. Statistical analysis was performed using the SAS 9.4 software program. ANCOVA was used to evaluate differences between test and control groups in new prescription volumes. Fluoroquinolone prescription volumes in the pretest period were used as a covariate in the ANCOVA model to adjust for pretest period prescription writing differences between the two groups. Program success was determined using a difference of adjusted means test with a p-value <0.05. All test versus control group differences were measured using a two-tail statistical testing method. Average differences in new prescription volumes were compared between participants receiving targeted short-form messages only, CME only, and targeted short-from messages combined with CME, using pairwise t-tests.
The impact of the total program (exposure to a targeted short-form message or CME) was determined as program-level new prescription volume and Prescriber Reducer differences. Test and control matches were time-aligned by the first program engagement to synchronize follow-up program periods.
Following program completion, analysis of program impact on new prescription volume by indication for ABS, ABECB-COPD, and uUTI was performed by IQVIA using ANCOVA for patient medical claims data. As medical claims data has varying levels of coverage, IQVIA groups HCPs in three tiers for indication tracking, as defined in Table 1. Program participant fluoroquinolone prescribing volume changes by indication were analyzed by their tier membership. Only HCPs with patients with a relevant indication were included in the analysis.
Table 1. HCP medical claims data coverage
| Tier | Claims Coverage* |
|---|---|
| Tier 1 | HCPs whose claims were considered 100% captured in IQVIA Medical data in every month of the test/posttest period (November 2016 - July 2017) |
| Tier 2 | HCPs whose claims were considered less than 100% captured in IQVIA Medical data in some of the months of the test/posttest period (November 2016 - July 2017) |
| Tier 3 | HCPs who are not classified in tier 1 and 2 in the test/post test period (November 2016 - July 2017) |
Ethics and confidentiality
Neither the overall program nor the impact study recorded any protected information as defined in the 1996 Health Insurance Portability and Accountability Act (HIPAA). The study complied with Medscape's privacy policy as posted on medscape.com. IQVIA used patient data that were de-identified to ensure privacy and compliance with HIPAA and other relevant privacy legislation. Presentation of individualized prescribing data and practice patterns to prescribers was also de-identified and HIPAA-compliant. No specific permission from prescribers or patients was required to perform this education and analysis program.
RESULTS
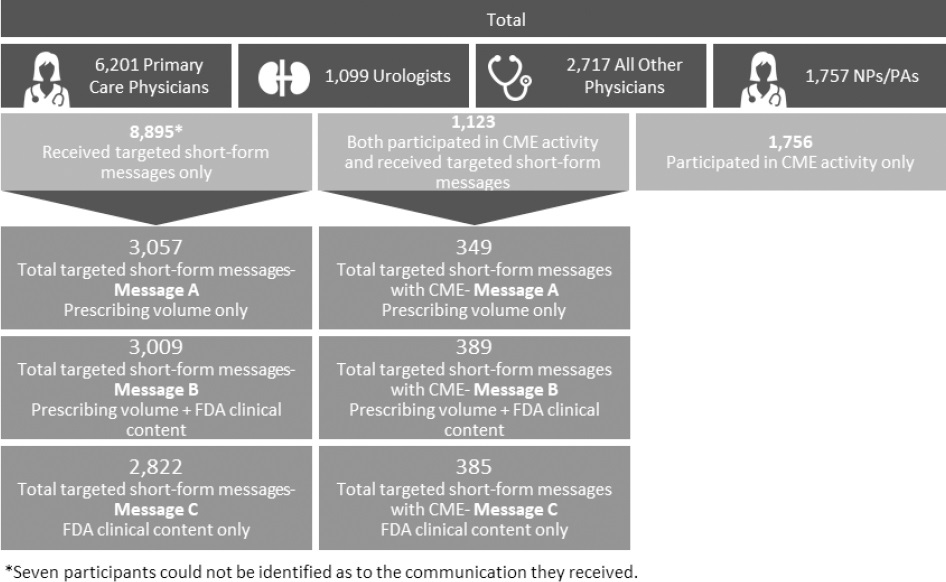
The test group of HCPs, all matched to controls, consisted of 6201 primary care physicians, 1099 urologists, 2717 all other physicians, and 1757 nurse practitioners/physician assistants. Among the test group, 8895 participants received targeted short-form messages only, 1756 received CME only, and 1123 received both targeted short-form messages and CME. Figure 2 shows the distribution breakdowns for targeted short-form messages A, B, and C individually, and combined with CME.
On a program level across all three tactics, the test group showed a change in new prescription volume of -10.3% compared with the control group. Test group participants receiving targeted short-form messages alone, CME alone, and targeted short-form messages combined with CME showed a change in new prescription volume of -8.5%, -12.3%, and -21.7%, respectively, compared with the control group (Table 2).
Table 2. Oral fluoroquinolone new prescription volume comparison: total and by tactic
| Tactic/Message | Test Cohort (n) | New prescription volume percent difference compared with control (95%CI) | p-value |
|---|---|---|---|
| All Tactics | Total (n=11,774) | -10.3% (-11.8%, -8.8%) | p<0.0001 |
| Targeted short-form messages participants | Total (n=8,895) | -8.5% (-10.2%, -6.9%) | p<0.0001 |
| Message A*: Prescriber's fluoroquinolone prescription volume compared with national average for specialty (n=3,057) | -7.9% (-10.7%, -5.2%) | p<0.0001 | |
| Message B*: Prescriber's fluoroquinolone prescription volume compared with national average for specialty, combined with FDA advisory on appropriate (n=3,009) | -9.4% (-12.0%, -6.7%) | p<0.0001 | |
| Message C*: FDA advisory on appropriate fluoroquinolone prescribing (n=2,822) | -8.2% (-11.3%, -5.1%) | p<0.0001 | |
| CME | CME Only Participants (n=1,756) | -12.3% (-19.2%, -5.5%) | p=0.0004 |
| Both CME and Targeted Short-Form Messages Participants (n=1,123) | -21.7% (-26.6%, -16.9%) | p<0.0001 |
*There are no significant differences in level of impact resulting from exposure to Message A, B, or C (based on a pairwise comparison t-test)
Participants receiving targeted short-form messages A, B, or C showed a change in new prescription volume versus controls of -7.9% (p<0.0001), -9.4% (p<0.0001), -8.2% (p<0.0001), respectively (Table 2). There was no significant difference in level of prescribing volume resulting from exposure to one message versus another.
Program participation resulted in a significant (p<0.0001) reduction in new prescription volume for the test group (80.1%; 9427/11,774) as compared with the control group (76.2%; 8966/11,774). Test specialty subgroups, as compared with control specialty subgroups, showed a reduction in new prescription volume for PCPs of 4.0% (250/6,201; p<0.0001); urologists, 6.4% (70/1,099; p=0.0002); and all other MDs, 3.6% (98/2,717; p=0.0028). Only nurse practitioners/physician assistants did not show a significant reduction (2.4%; 43/1,757; p=0.0904).
New prescription volume for test participants versus controls decreased significantly across Tiers 1, 2, and 3 for ABS (12.8%) and uUTI (9.0%) following program participation (Table 3). Prescribing volume for ABECB-COPD was not significantly reduced. The differences between indications were not statistically significant: ABS vs. ABECB p=0.3325; ABS vs. uUTI p=0.5686; and ABECB-COPD vs. uUTI p=0.1414.
Table 3. Oral fluoroquinolone new prescription volume comparison (all tactics): by tier* and reverent indication
| Tier* | Indication | New prescription volume percent difference compared with control (95% CI) | p-value |
|---|---|---|---|
| Tier 1 | Acute Bacterial Sinusitis (ABS) (Test n=1,319, Control n=1,381) | -10.6% (-23.6%, 2.4%) | p=0.1091 |
| Acute Bacterial Exacerbations of Chronic Bronchitis in people with Chronic Obstructive Pulmonary disease (ABECB-COPD) (Test n=1,161, Control n=1,210) | -7.5% (-20.4%, 5.5%) | p=0.2586 | |
| Uncomplicated Urinary Tract Infection (uUTI) (Test n=1,489, Control n=1,544) | -5.5% (-11.0%, -0.1%) | p=0.0470 | |
| Any combination of the three indications (ABS, ABECB-COPD or uUTI) (Test n=1,555, Control n=1,622) | -6.7% (-12.1%, -1.3%) | p=0.0159 | |
| Tiers 2+3 | Acute Bacterial Sinusitis (ABS) (Test n=6,500, Control n=6,412) | -14.0% (-23.8%, -4.3%) | p=0.0049 |
| Acute Bacterial Exacerbations of Chronic Bronchitis in people with Chronic Obstructive Pulmonary disease (ABECB-COPD) (Test n=5,027, Control n=4,830) | -3.5% (-11.6%, 4.6%) | p=0.3993 | |
| Uncomplicated Urinary Tract Infection (uUTI) (Test n=7,957, Control n=7,769) | -10.0% (-14.8%, -5.3%) | p<0.0001 | |
| Any combination of the three indications (ABS, ABECB-COPD or uUTI) (Test n=8,508, Control n=8,289) | -10.0% (-14.6%, -5.5%) | p<0.0001 | |
| Total (Tiers 1, 2, and 3) | Acute Bacterial Sinusitis (ABS) (Test n=7,819, Control n=7,793) | -12.8% (-20.7%, -4.9%) | p=0.0015 |
| Acute Bacterial Exacerbations of Chronic Bronchitis in people with Chronic Obstructive Pulmonary disease (ABECB-COPD) (Test n=6,188, Control n=6,040) | -4.5% (-11.4%, 2.5%) | p=0.2061 | |
| Uncomplicated Urinary Tract Infection (uUTI) (Test n=9,446, Control n=9,313) | -9.0% (-12.8%, -5.1%) | p<0.0001 | |
| Any of the three indications (ABS, ABECB-COPD or UTI) (Test n=10,063, Control n=9,911) | -9.2% (-12.8%, -5.5%) | p<0.0001 |
*See Data Analysis in Methods section and Table 1 for definitions of tiers.
Emails of targeted short-form messages containing comparative prescribing information, with and without clinical context, were opened at slightly higher rates (10.6%; 2659/25,043 for Message A and 10.8%;2682/24,883 for Message B) than targeted short-form messages containing clinical context alone (9.1%;2318/25,470 for Message C). Denominators for these calculations were the sum of all four waves of emails for each message.
DISCUSSION
To help answer persistent questions regarding the degree of effectiveness associated with different feedback formats designed to change clinical behaviors—and how best to optimize the effect of feedback interventions — we evaluated the effect of targeted short-form messages or CME on prescribing of fluoroquinolones among high prescribers. Studies suggest that multifaceted approaches are likely to be most effective in behavior change, such as combining comparative data on clinical practice with education.19 20 21-22Results from this ex post factomatched control study show that exposure to targeted short-form messages (with and without personalized feedback) and CME significantly reduced fluoroquinolone new prescription volume among PCPs, urologists, and all other physician providers. While targeted short-form messages and CME reduced new prescription volume by 8.5% and 12.3%, respectively (p<0.0001), pairing both targeted short-form messages and education yielded the greatest impact in reducing new prescription volume, by 21.7% (p<0.0001). The difference between this combination and each tactic alone is significant at p<0.0001. Accordingly, to optimize the effect of feedback interventions, education can be considered as a valuable complement to feedback that may provide a synergistic effect on outcomes.
The increased effectiveness of messaging combined with CME, as compared with messaging alone, is suggested by another recent study that showed a 29% decrease in fluoroquinolone prescribing among high-volume antibiotic prescribers who received peer comparison reports that included education about ways to reduce fluoroquinolone utilization for common diseases such as lower respiratory tract infections and asymptomatic bacteriuria.23The study investigators cited the educational feature as a possible explanation for the positive results achieved compared with a Swiss study that did not show any differences in outpatient antibiotic utilization after an intervention that provided peer prescriber comparison reports — but did not provide any educational resources — to prescribers.24
Our results also suggest that targeted short-form messages paired with online CME may be a viable alternative to other tactics that, depending on the systems that may or not be in place to carry out these tactics, are thought to be more time-consuming (e.g., carbon copy prescription pads that require collection and evaluation; telephone interviews with patients; day-long, small-group, academic detailing meetings plus follow-up meetings; point of care clinical decision support tools which require several weeks training), expensive (e.g., electronic clinical decision support systems; incentive payments for program participation), and not easily scalable (small group training sessions in communication skills; practice profiling for prescribing rates) drivers of practice change.10,19,25 26 27 28 29 30 31 32 33 34-35
Although not directly linked to outcomes measures for new prescription volume or Prescriber Reducers, we observed that targeted short-form messages with subject lines addressing personal fluoroquinolone prescribing (Messages A and B) were opened at slightly higher rates (10.6% and 10.8%, respectively) than targeted short-form messages with a subject line addressing fluoroquinolone safety data only (Message C, 9.1%). This result may suggest that feedback on personal prescribing had a somewhat greater effect on physician engagement than safety data alone and, by extension, was more likely to lead HCPs to education that may influence prescribing behavior.
The effectiveness of feedback on personal prescribing was recently demonstrated by a study in which the Chief Medical Officer for England mailed letters providing personal prescribing feedback to high-prescribing practices (n=791), resulting in a 3.3% reduction in the antibiotic prescribing rate compared with a control group (which received no letter) over a six-month period.36However, in contrast to our findings and those from the aforementioned study in England, a recent study by another government agency—the Centers for Medicare and Medicaid Services (CMS)—testing an intervention to reduce opioid prescribing reported an inability to detect a statistically significant effect of a letter on personal prescribing behavior sent to high prescribers from CMS; likely reasons cited for the failure to detect an effect involved whether the letters reached their intended targets and whether the letters, even if appropriately targeted, were effective at altering behavior.25
Interestingly, another recent study tested three digitally delivered interventions to reduce antibiotic use in acute respiratory infections among top prescribers in 47 primary care practices in Boston and Los Angeles (n=248).37The interventions included a suggested alternative therapy presented to prescribers via electronic health records (EHRs); an invitation to enter a free-text justification of antibiotic selection in the EHR; and an email providing peer comparison of prescribing behavior. Of the three interventions, peer comparison and justification resulted in decreased prescribing (-18.1% and -16.3%, respectively).
While further studies will be necessary to establish the degree of effectiveness of digital (emails, EHR, online education) versus analog (in-person small-group meetings and training sessions, carbon copy prescription pads, letters, telephone interviews) interventions, in our study the reduced new prescription volume among high-volume fluoroquinolone prescribers receiving targeted short-form messages and CME suggests that each of these online tactics may be superior to analog tactics as interventions for reducing prescribing.
It is also important to consider that many of the studies examining audit and feedback for antibiotic prescribing consist of relatively small sample sizes.13 Our capacity to access a large volume of practitioner-level prescribing data and deliver comparative prescribing information to a large population of practitioners is a major strength of our study and has direct implications for impact on public health.
Possible limitations of our study are the inability to determine if all reductions in prescribing were for inappropriate uses; the inability to control for the potential influence of local antimicrobial stewardship programs or influences other than the intervention studied here; the inconsistent nature of Tier 2 and 3 medical claims data used to evaluate new prescribing volume by indication; the short-term nature of this study; the timing of our study in relation to the FDA safety alert; and the inability to evaluate whether reduced prescribing associated with Message C is a durable result, as the subject line of this message (“Serious side effects prompt FDA to change fluoroquinolone labeling”) may provide an increased social incentive to respond due to participants' familiarity with the FDA. Finally, in contrast to many other studies where feedback is delivered by a peer or an employer, this intervention was delivered by Medscape, an online provider of medical education and news with a large membership database. Medscape's ubiquity in the medical community may present a challenge in distinguishing whether the effects attributed to our study interventions were not to some degree associated with the intervention provider. Additional studies will be needed to determine if this approach is equivalent to that delivered by a peer or employer, and to evaluate the applicability of our study results to other classes of drugs without labelling changes or safety warnings to attempt to isolate specific factors most responsible for driving the observed behavior change.
Several factors could explain our findings. We targeted a single behavior (antibiotic prescribing), addressed a measurable outcome (number of fluoroquinolone prescriptions), and communicated a clear action to test subjects (i.e., avoid prescribing fluoroquinolones for particular indications when there is an alternative).12,16 We also designed and targeted the intervention to have the greatest potential effect. HCPs with lower baseline compliance with the desired outcome (i.e., higher prescribers of fluoroquinolones) were selected for the intervention, as reviews of audit and feedback trials have demonstrated that low baseline performance is associated with a larger effect.12,16
CONCLUSIONS
Our study, conducted as part of an FDA initiative to reduce preventable harm from drugs, shows that targeted short-form messages on appropriate prescribing of oral fluoroquinolones reduced fluoroquinolone prescribing among high prescribers, and email targeted short-form messages with subject lines addressing personal fluoroquinolone prescribing were opened at a slightly higher rate than targeted short-form messages with subject lines addressing fluoroquinolone safety data only. Targeted short-form messages and CME each resulted in significant new prescription volume reduction versus control. Combining targeted short-form messages with CME yielded the greatest percentage of HCPs with reduced fluoroquinolone prescribing (80.1% for test versus 76.2% reduction for matched control [p<0.0001]). Targeted short-form messages paired with online CME is a fast-acting, easily implemented intervention for reducing prescribing of fluoroquinolones.