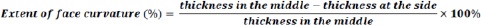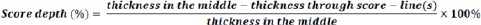INTRODUCTION
Tablet subdivision can be used by patients for a variety of reasons.1 2-3 These reasons include reduced doses of the medication, smaller parts of the tablets to ease swallowing, and/or economic factors for which subdividing a higher strength drug product might be less expensive during the course of therapy. However, subdividing tablets might not be a recommended practice. Tablets for controlled release purposes and orally disintegrating tablets might lose predesigned properties when subdivided.4 5-6 In addition, tablet subdivision might result in two halves that are different in weight and in the corresponding amount of active ingredient.7 8-9 This can have a serious consequence for potent drugs with a narrow therapeutic index.1,9 10-11 In an ideal situation, a split tablet should result in two halves that have equal weights and contain the same content of the drug. However, in real life practice, there is a variation in the degree of accuracy in obtaining equal halves.8 Several factors could influence this accuracy such as the presence of scores on the tablet surfaces, depth of the scores, surface flatness of the tablet, tablet size, tablet shape, tablet crushing strength (hardness), tablet composition (excipients), and the technique used for subdivision of the tablet.2,7,9,12 13-14 The presence of scores on a tablet surface could increase the chance of obtaining accurate subdivision especially if the scores are deep and are present on both faces.13 In some cases the presence of scores can be misleading if they are not deep enough to facilitate subdivision.7 Tablets with flat surfaces are expected to be more accurately subdivided into two equal halves compared to tablets with curved surfaces.7 In addition, tablets that are oblong are expected to be more accurately subdivided.12 Researchers reported that the choice of excipients such as fillers and binders affected the accuracy of tablet subdivision.14 They found that dicalcium phosphate dihydrate produced tablets with higher subdivision accuracy compared to tablets made of microcrystalline cellulose. However, combination of the binder hydroxypropylcellulose with microcrystalline cellulose in the formulation improved the subdivision accuracy of the tablets.14 Several techniques such as by hand, by knife, by tablet cutter, or by teeth can be used in order to subdivide tablets.15
Several studies have investigated the accuracy of different tablet subdivision techniques.7,8,16 For example, van Riet-Nales et al. investigated the accuracy of tablet subdivision associated with the use of three subdivision techniques. Researchers compared the results of subdividing paracetamol 500 mg tablets which were round, flat, and uncoated obtained by tablet splitter (cutter), kitchen knife, and hand. They found that the accuracy of tablet subdivision was best achieved by hand.8 Moreover, Habib et al. studied the accuracy of tablet subdivision of salbutamol tablets by hand and tablet cutter.16 The results showed that the use of a tablet cutter is superior to hand subdivision in producing equal half tablets.
In 2017 the tablet subdivision practices in Jordan as well as the frequency of using different techniques for tablet subdivision were investigated.15 The results showed that the majority of participants (63.5%) subdivided their drug products by hand, followed by kitchen knife (14.3%) and tablet cutter (9.0%). In addition, It was found that warfarin 5 mg, levothyroxine 50 μg, levothyroxine 100 μg, candesartan 16 mg, and carvedilol 25 mg are among the ten most commonly subdivided drug products.15 The objective of this study was to investigate the accuracy, variability, and weight uniformity of half tablets produced by subdividing five commonly split medications in Jordan by different tablet subdivision techniques.
METHODS
The study protocol was approved by the Jordan University of Science and Technology Institutional Review Board (Research Number 8/101/2016) on 15 December 2016.
Tablets of five commonly subdivided medications in Jordan were used in this study. These drug products were warfarin sodium 5 mg (Orfarin® 5 mg, Lot #1793373, expiry date 06-2019; Orion Corporation, Finland), levothyroxine 100 μg (Euthyrox® 100 μg, Lot #244669, expiry date 10-2020; Merck KGaA, Germany), levothyroxine 50 μg (Euthyrox® 50 μg, Lot #247655), expiry date 12-2020; Merck KGaA, Germany), candesartan 16 mg (Blopress® 16 mg, Lot #756097, expiry date 04-2021; The Arab Pharmaceutical Manufacturing Co. Ltd., Jordan), and carvedilol 25 mg (Carvidol® 25 mg, Lot #18111, expiry date 02-2021; Pharma International Co., Jordan). Tablet dimensions were determined using a Vernier caliper. The thickness and diameter of ten tablets of each drug product were measured. The thickness of bi-convex tablets was measured in the center and on the side of the tablet. The difference was used as an indication of the extent of face curvature.
In addition, the thickness was measured from inside the score-line(s) if present on the tablet and used as an indication of score depth.
The crushing strength of tablets was analyzed using a hardness tester (Biobase Tablet Hardness Tester YD-3, Jinan, China). Ten tablets of each drug were analyzed. A 32-year-old right-handed female pharmacist volunteered to carry out all tablets subdivisions. Ten tablets of each drug product were subdivided by each of the studied subdivision techniques: by hand, by kitchen knife, and by a tablet cutter. The weight of each tablet and the resulting subdivisions were measured using a digital balance (Phoenix Instument-ASN324, Garbsen, Germany). All subdivisions were performed along the score-line if present. Subdivision by a kitchen knife was performed by placing the tablet on the bench top, placing the sharp side of the blade along the score-line, if present, and then pressing on the non-sharp end of the blade in one hand while holding the handle in the other hand. The kitchen knife blade was made of stainless steel with a length of 7.8 cm, and the blade thickness was 1.0 mm measured midway of the blade. The handle of the knife was made of plastic with a length of 11.4 cm, and the other two dimensions were 1.4 cm and 2.1 cm measured midway of the handle. The tablet cutter used in this study was a non-brand plastic splitter commonly available in the market in Jordan. The dimensions of the tablet cutter box were 7.0 cm, 2.9 cm, and 2.5 cm. The metal blade of the cutter had a thickness of 0.30 mm at the middle point. The tablets were placed at the closest point towards the hinge of the cutter inside the designated area on the base plate of the cutter which was parallel to the horizontal plane along the x-axis. This designated area resembled an oval in shape with axis of symmetry (major axis) along the direction of the cutting blade with a length of 3.0 cm. The wider region of the oval shaped area laid closer towards the hinge of the cutter. The maximum width of the oval region was 2.0 cm. This designated area had a wall (ridge) of a height of 4.0 mm all around it except for the side closest to the hinge of the cutter along the major axis. This opening in the wall had a width of 4.0 mm and was centered under the point where the cutting blade starts cutting the placed tablets. The distance between the hinge and the wall opening is equal to 1.25 cm. With this geometry, it is expected that the radii of tablets tested in this study had little effect on the distance between the hinge of the tablet cutter and the point at which the cutting blade starts acting on the placed tablets. Somogyi et al. proposed schemes of forces during tablet breaking for tablets of different shapes.17 The geometry of the used device could influence these schemes. However, we anticipate that the scheme of forces during tablet breaking for all tested tablets in this study was similar to what was depicted by Somogyi et al. for a conventional splitting device available in the European market for round tablets.17 Accuracy and variability (precision) of tablet subdivision were evaluated according to the suggestions of van Riet-Nales et al.8
In addition, a two-tailed Welch's t-test statistical analysis (significance level=0.05) was performed to assess the means of % weights of the smaller tablet fractions produced by the applied subdivision techniques for each drug product. Accordingly, the means of % weights of the smaller fractions subdivided by the tablet cutter were assessed in relation to those subdivided by hand and those subdivided by knife for each drug product. In addition, the means of % weights of the smaller fractions subdivided by hand were assessed in relation to those subdivided by the knife for each drug product. A weight uniformity test was performed on half tablets produced by the subdivision techniques applied in this study. The weight uniformity test for half tablets was applied according to the suggestions made by Polli et al. which was adapted from the U.S. Pharmacopeia's (USP) <905> “Uniformity of Dosage Units” test for whole tablets.18,19 Accordingly, in this study 30 tablets of each drug product were weighed individually and the average weight was calculated. The average weight was divided by two to obtain an average weight for a uniformly subdivided half tablet. Ten tablets of each drug product were subdivided using one of the subdivision techniques applied in this study. The produced half tablets were weighed individually. The half tablets passed the test if no more than one half tablet of the produced twenty parts weighed less than 85% or greater than 115% of the average weight of the uniformly subdivided half tablet and no half tablet weighed less than 75% or greater than 125% of the average weight of the uniformly subdivided half tablet. In addition, the RSD (relative standard deviation) for the weights of the obtained 20 parts (half tablets) should be less than or equal to 10%.
If two half tablets weighed less than 85% or greater than 115% and no half tablet was less than 75% or greater than 125% of the average weight of the uniformly subdivided half tablet or if the RSD was greater than 10%, then the other 20 tablets were subdivided to produce an additional 40 parts which were individually weighed. The tablet halves were accepted if out of the 60 parts only two half tablets weighed outside the range of 85%-115% of the average weight of the uniformly subdivided half tablet and no half tablet was outside the range of 75%-125% of the average weight of the uniformly subdivided half tablet. In addition, the RSD should be less than or equal to 10%. The half tablets were rejected if more than two tablets weighed outside the range 85%-115%, if a half tablet was outside the range 75%-125% of the average weight of the uniformly subdivided half tablet, or if the RSD was greater than 10%. Similar testing criteria for weight uniformity adapted from the European Pharmacopoeia were used by other researchers.8 In addition, the % weight of subdivided tablets were plotted against their number. Horizontal lines corresponding to the 85% and 115% weight limits were added to the plots in order to represent the individual tablet subdivision % weight in relation to these acceptance limits.
RESULTS
Table 1 shows the properties of the subdivided tablets of the studied drug products. All tablets had a round shape. The average weights of these tablets were between 100.63 mg (SD=0.99) and 379.04 mg (SD=3.00). Tablets of warfarin, levothyroxine 50 μg, levothyroxine 100 μg, and candesartan had relatively similar diameters. However, their thicknesses varied according to the corresponding weight. Carvedilol tablets had the largest weight, diameter, and thickness. In addition, they were the only ones without scores. Tablets of levothyroxine 50 μg and levothyroxine 100 μg had scores on both sides. Both candesartan and carvedilol tablets were bi-convex in shape with a similar extent of face curvature. Warfarin had the highest percent score depth among these tablets. The tablets had crushing strength values that ranged between 23.29 N (SD=3.58) and 103.35 N (SD=14.98). Candesartan tablets had the lowest crushing strength while carvedilol tablets had the highest crushing strength.
Table 1. Tablet properties of drug products used in the study (n=10)
| Drug product | Tablet weight | Tablet diameter | Thickness in the middle | Extent of face curvature (%) | Score depth (%) | Crushing strength (N) | Tablet shape | Presence of score-line |
|---|---|---|---|---|---|---|---|---|
| Warfarin | 136.7 SD=1.58 | 7.05 SD=0.04 | 2.97 SD=0.02 | 0 | 23.77 SD=1.57 | 39.24 SD=5.01 | Round | One side |
| Levothyroxine 50 μg | 100.93 SD=1.32 | 7.13 SD=0.03 | 2.20 SD=0.04 | 0 | 21.63 SD=2.96 | 49.87 SD=7.22 | Round | Both sides |
| Levothyroxine 100 μg | 100.63 SD=0.99 | 7.12 SD=0.03 | 2.22 SD=0.03 | 0 | 22.97 SD=2.24 | 41.00 SD=9.56 | Round | Both sides |
| Candesartan | 130.55 SD=3.19 | 7.18 SD=0.05 | 3.14 SD=0.07 | 33.64 SD=3.62 | 10.98 SD=2.69 | 23.29 SD=3.58 | Round | One side |
| Carvedilol | 379.04 SD=3.00 | 10.52 SD=0.03 | 3.98 SD=0.04 | 30.94 SD=2.45 | 0 | 103.35 SD=14.98 | Round | Non-scored |
SD: standard deviation
Table 2 shows the accuracy of tablet subdivision of the smaller and larger halves produced by different techniques. It can be seen that the accuracy range for the smaller fractions was between 81.0% and 95.2% for all techniques applied in this study. Moreover, the accuracy range for the larger fractions was between 97.5% and 109.8%. In addition, Table 2 shows that the range of the RSD was between 2.0% and 17.4% for the smaller fractions and was between 1.4% and 7.7% for the larger fractions. According to the Welch's t-test statistical analysis, there was a significant difference between the means of % weights of the smaller fractions of subdivided tablets in the following cases: subdivision by the tablet cutter compared to subdivision by hand for levothyroxine 50 μg (p=0.003) and candesartan (p=0.022) tablets and subdivision by the tablet cutter compared to subdivision by knife for levothyroxine 50 μg (p=0.001) and levothyroxine 100 μg (p=0.041) tablets.
Table 2. Average accuracy (%) and relative standard deviation (RSD) of tablet subdivision of the subdivision techniques
| Fraction | Average accuracy (%) by hand | RSD by hand (%) | Average accuracy (%) by cutter | RSD by cutter (%) | Average accuracy (%) by knife | RSD by knife (%) |
|---|---|---|---|---|---|---|
| Warfarin | ||||||
| Small | 90.7 | 9.0 | 89.8 | 15.7 | 87.4 | 8.0 |
| Large | 103.9 | 4.8 | 101.7 | 1.8 | 103.6 | 7.7 |
| Levothyroxine 50 μg | ||||||
| Small | 89.7 | 3.2 | 93.9 | 2.6 | 86.3 | 6.2 |
| Large | 106.5 | 2.7 | 102.0 | 2.7 | 100.0 | 5.1 |
| Levothyroxine 100 μg | ||||||
| Small | 90.3 | 2.0 | 91.7 | 3.3 | 81.0 | 17.4 |
| Large | 104.8 | 1.4 | 103.2 | 2.9 | 97.5 | 3.7 |
| Candesartan | ||||||
| Small | 88.8 | 7.6 | 95.0 | 3.2 | 93.1 | 2.5 |
| Large | 109.8 | 6.1 | 103.4 | 2.9 | 102.4 | 3.5 |
| Carvedilol | ||||||
| Small | 87.8 | 13.9 | 95.2 | 4.3 | 91.7 | 8.3 |
| Large | 108.3 | 5.1 | 103.8 | 4.0 | 107.0 | 7.1 |
A summary of the results of the weight variation test performed on the half tablets produced by the different subdivision techniques is shown in Table 3. No subdivision technique used in this study produced warfarin half tablets that passed the test of weight variation. However, subdividing tablets of levothyroxine 50 μg and levothyroxine 100 μg by hand and by the tablet cutter produced half tablets that passed the test. Subdividing tablets of candesartan by the tablet cutter and by knife produced half tablets that passed the test. Moreover, subdividing carvedilol tablets only by the tablet cutter produced half tablets that passed the test.
Table 3. Summary of weight variation test results for half tablets produced by the different subdivision techniques
| Subdivision technique | Half tablets outside 85%-115% and within 75%-125% (n=20) | Number of half tablets outside 75%-125% (n=20) | RSD (%) (n=20) | Result |
|---|---|---|---|---|
| Warfarin | ||||
| Hand | 1 | 1 | 9.7 | Reject |
| Cutter | 0 | 1 | 12.1 | Reject |
| Knife | 5 | 0 | 11.5 | Reject |
| Levothyroxine 50 μg | ||||
| Hand | 1 | 0 | 9.3 | Accept |
| Cutter | 0 | 0 | 5.3 | Accept |
| Knife | 3 | 0 | 9.1 | Reject |
| Levothyroxine 100 μg | ||||
| Hand | 0 | 0 | 3.8 | Accept |
| Cutter | 0 | 0 | 6.9 | Accept |
| Knife | 3 | 2 | 14.6 | Reject |
| Candesartan | ||||
| Hand | 4 | 1 | 12.7 | Reject |
| Cutter | 0 | 0 | 5.4 | Accept |
| Knife | 0 | 0 | 5.8 | Accept |
| Carvedilol | ||||
| Hand | 2 | 1 | 14.2 | Reject |
| Cutter | 0 | 0 | 6.1 | Accept |
| Knife | 4 | 0 | 11.0 | Reject |
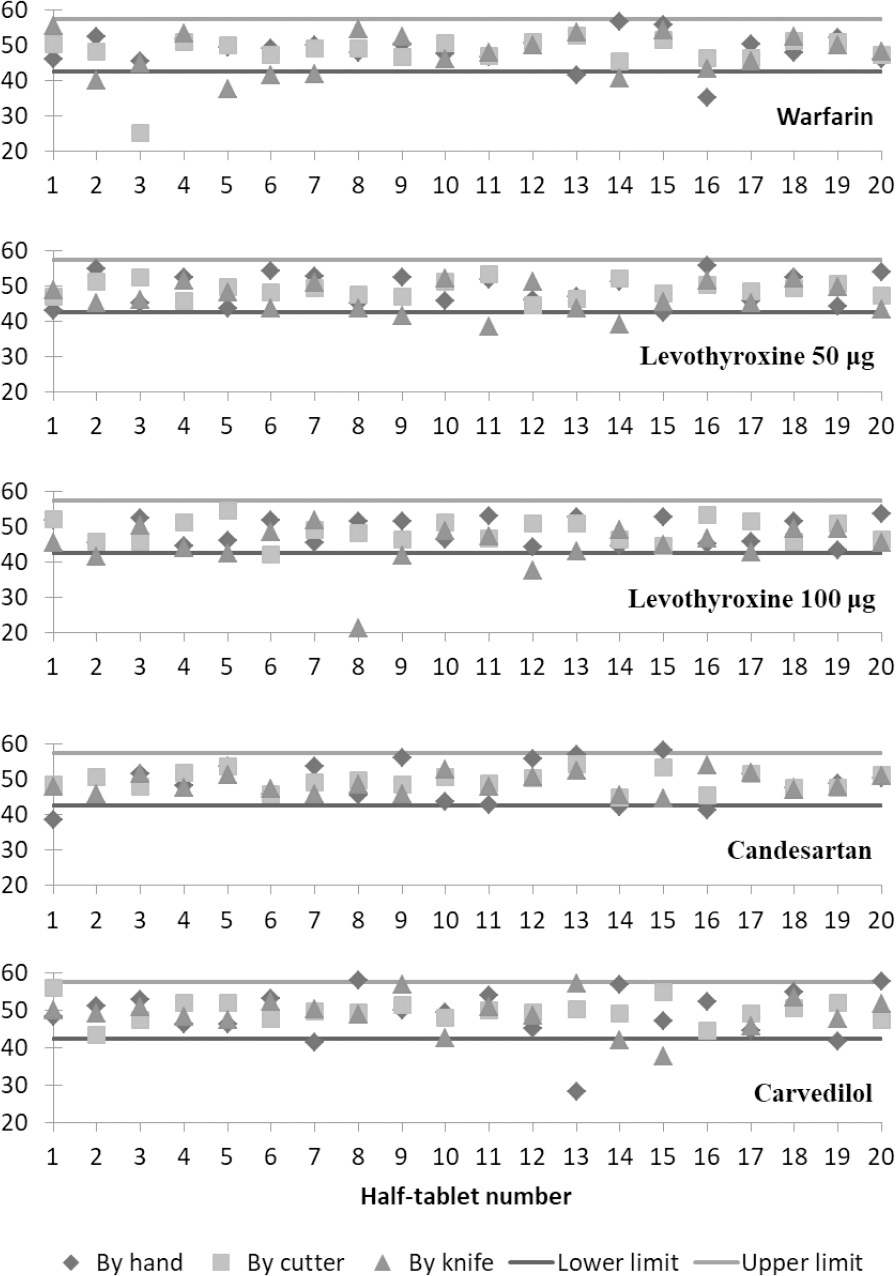
Figure 1 shows plots of the percentage weights of the 20 tablet divisions of each drug product produced by the applied subdivision techniques. The lower and upper horizontal lines in each plot represent the acceptance limits of 85% and 115 % of the weights of perfectly subdivided half tablets.
DISCUSSION
This was the first study to investigate the accuracy of tablet subdivision using different subdivision techniques for five of the most commonly subdivided drug products in Jordan.15
The tablets of the five drug products used in this study varied in terms of weight, diameter, thickness, presence of score, score depth, face curvature, and crushing strength. The closest average percent of accuracy to being perfectly subdivided of the smaller fractions was obtained for carvedilol tablets subdivided by the tablet cutter (Table 2). In this case, the larger fractions of subdivided carvedilol tablets had an average percent accuracy of 103.8 and the subdivided tablets passed the weight variation test (Table 3). This is probably due to the larger size of the tablets which facilitated more accurate subdivision using the tablet cutter. Thus, a score-line was not necessary to obtain an accurate tablet subdivision using the tablet cutter. In addition, the closest average percent of accuracy to being perfectly subdivided of the larger fractions was obtained for levothyroxine 50 μg tablets subdivided by knife. However, the smaller fractions of subdivided levothyroxine 50 μg tablets had an average percent accuracy equal to 86.3 and the subdivided tablets did not pass the weight variation test (Table 3). It is important to note that patients might use either the smaller or the larger subdivided fraction and discard the other, or might use one subdivided fraction of the tablet and save the other fraction for later use. Therefore, it is important to consider the accuracy of subdivision for both fractions. In addition, the least variability (lowest RSD value) for the percent accuracy values of the smaller subdivided fractions was obtained for levothyroxine 100 μg subdivided by hand. This is probably due to the fact that these tablets had score-lines on both faces of the tablets, the tablets faces were flat, and their percent score depth was relatively high.
The results of the weight uniformity test used in this study show that the acceptance of the weights of produced half tablets depends upon both tablet properties and used subdivision technique. For example, warfarin half tablets failed the test regardless of the applied subdivision technique. Warfarin tablets were scored on one side, had flat faces, and a score depth of 24% which seems to be suitable for tablet subdivision. However, results of the weight uniformity test were opposite to that (Table 3). Moreover, Figure 1 shows a number of subdivided tablets of warfarin beneath the 85% limit line for all of the applied subdivision techniques. This could be due to the fact that these tablets had a relatively wide score line. The score line on warfarin tablets was about twice the thickness of the knife's blade and several times the thickness of the cutter's blade. Even for subdivision by hand, having a wide score line on tablets could lead to increased probability of having unequal and variable subdivisions. Interestingly, the results of the current study differ from the results of a previously published study that found that subdividing warfarin tablets by a kitchen knife produced weight-uniform half tablets although both batches were produced by the same manufacturer.7 This discrepancy can be attributed to differences in tablet thickness and hardness. According to the previously published study, the thickness and hardness (crushing strength) of studied warfarin tablets were 2.86 mm (SD=0.01) and 68.9 N (SD=3.4), respectively. In addition, these tablets had a percentage score depth of 28%.7 Therefore, tablets of the previously tested batch had less thickness, higher percentage score depth, and higher tablet hardness. The aforementioned finding indicates that even with the same manufacturer, the practice of subdividing tablets may or may not result in acceptable subdivisions. In addition, different brands of warfarin tablets are expected to show dissimilar results due to variations in tablet properties. For example, subdivisions of Coumadin® 5 mg tablets, which are round, scored, and non-flat, passed the weight uniformity test set by the researchers when subdivided by a tablet cutter.18
Figure 1 shows the distribution of half tablet % weights of levothyroxine 50 μg and levothyroxine 100 μg compared to the 85-115% limits. Levothyroxine 50 μg and levothyroxine 100 μg half tablets were accepted in the weight uniformity test (Table 3) for subdivision by hand and by the tablet cutter. However, it can be seen that a number of half tablets were under the 85% limit for subdivision by knife. Other researchers investigated the subdivision of tablets of other marketed brands of levothyroxine by hand and by a tablet cutter.11They found that the two applied subdivision methods produced subdivided tablets that failed the content uniformity test at a rate higher than that for whole tablets. Variations in the formulation aspects in addition to other pre-mentioned variations in tablet properties and properties of the tablet cutter used could lead to different acceptance results for different drug products of the same active ingredient. Several of the % weights of the produced half tablets subdivided by hand were outside the acceptable 85-115% limits for candesartan tablets (Table 3 and Figure 1). This is probably due to the fact that candesartan tablets are bi-convex in shape which makes them more difficult to be subdivided by hand. Another study investigating the uniformity of tablet subdivision by hand and by a tablet cutter of another brand of candesartan found that both of these techniques produced tablet subdivisions that failed the content uniformity test.20Investigated tablets of candesartan 16 mg were round and scored on one side and had a similar size to the tablets used in this study.
Carvedilol tablets were bi-convex in shape and non-scored which resulted in failing the uniformity test of half tablets produced by hand and by knife (Table 3). Accordingly, Figure 1 shows the % weights of a number of subdivided half tablets outside the 85-115% limits for both by hand and by knife subdivision techniques. This finding does not coincide with what was suggested by Somogyi et al. regarding the method of choice for subdividing large unscored uncoated tablets (Algozone® tablets).17Somogyi et al. suggested that for such tablets a kitchen knife is the preferable subdivision technique. However, carvedilol tablets were bi-convex while those of Algozone® tablets were flat. This property would make the tablet more difficult to break by knife or by hand. A study on the subdivision of tablets by knife which included Dilatrend® tablets (carvedilol 25 mg) showed that the subdivision of the tablets of this drug product failed the weight uniformity test.9 However, it is important to note that these tablets were round, flat-faced, and scored on both sides and had a smaller size when compared to the carvedilol tablets used in this study.
Most patients in Jordan use their hands to subdivide tablets.15 However, the results of this study showed that hand subdivision produced half tablets that do not pass the weight uniformity test in the cases of warfarin, candesartan, and carvedilol. However, subdivision of tablets using the tablet cutter produced half tablets that were accepted for four of the five studied drugs in the current study.
Administering potent drugs with low therapeutic index as subdivided tablets can be of clinical significance. Thus, a small change in the dose can lead to the drug being ineffective or increasing the risk of side effects. All drugs tested in this study are potent and some with narrow therapeutic index (warfarin and levothyroxine). In addition, in most of cases these drugs are used chronically. Minimal dose change in one direction might lead to serious consequences. For example, if the patient routinely administers the larger subdivision fraction of the tablet and discards the other, then an unnecessary build up of plasma concentration might lead to subjecting the patient to additional risk of side effects. In addition, if the patient routinely administers the smaller fraction, then a failure to achieve a required therapeutic level might also arise. The pharmacists are required to educate patients about the best available method for subdivision of tablets. The current study has limitations. The subdivision of tablets was conducted by a pharmacist who was expected to be more accurate than the patient in performing the subdivisions. In addition, results may not be generalizable to patients with physical disabilities. Moreover, one kind of tablet cutter and kitchen knife was used, but different tablet cutters and kitchen knives could bring different results.
CONCLUSIONS
The effectiveness of subdivision techniques to produce uniform weights of half tablets varies according to tablet properties of the drug product and the subdivision technique. In this study, the tablet cutter seemed to be the most suitable technique for producing uniformly subdivided half tablets for all five studied drug products except for warfarin.