INTRODUCTION
Total knee replacement (TKR) is a major orthopedic surgery, which is considered high risk for the development of venous thromboembolism (VTE).1 Limiting both VTE events and bleeding episodes in this type of surgery is therefore essential. However, excessive anticoagulation should be avoided because it has a negative impact on the surgical outcome.2 In the last 10 years, there have been major changes in the delivery of orthopedic surgeries.3 This has included the implementation of strategies such as day surgery admission as well as the use of spinal anesthesia which has resulted in a reduction in the duration of operations.4 In addition, the use of appropriate analgesia allows early mobilization and aggressive rehabilitation, resulting in a mean length of hospitalization of 5 days.5 These strategies also contribute to decreasing the risk of death in the perioperative period and may also reduce incidences of VTE, which can be caused by restricted movement and prolonged length of stay and which were common after joint replacement surgeries.4,6
The annual incidence of VTE in the United States is estimated to range between 350,000 and 900,000, and approximately 100,000 die of the condition each year. Moreover, among those that survive, 30-50% will go on to develop post-thrombotic syndrome and as much as 30% will develop a second deep-vein thrombosis (DVT) within 5 years.7,8
To this end, the author designed a new risk stratification tool for TKR surgery after reviewing all of the related literature. Hence, all surgical and patient-related factors that show significant associations with the incidence of VTE events are included in the proposed risk stratification tool. The aim of this study is to assess the performance of the developed VTE risk stratification protocol by evaluating the clinical outcomes that resulted from the use of this new proposed protocol for selecting a suitable extended VTE prophylaxis for post TKR surgery patients administered in combination with patient education programs. It is hypothesized that the use of a new proposed VTE risk stratification protocol for selecting the extended VTE prophylaxis post TKR Surgeries along with patients’ educational programs will be able to decrease the complications post total knee replacement surgery. This research will answer the following question: Whether the use of a new proposed VTE risk stratification protocol for selecting the extended VTE prophylaxis post TKR Surgeries along with patients’ educational programs will be able to decrease the complications post total knee replacement surgery or not?
METHODS
In order to obtain the required data to test the proposed tool, a randomized controlled trial was conducted during the period of October 2018 to July 2019 in two medical centers in Saudi Arabia, namely, Prince Sultan Military Medical City (PSMMC) and the King Abd Allah University Hospital (KAAUH). The PSMMC is located in Riyadh and is considered to be one of the most advanced medical centers in the Middle East. It has a capacity of about 1,200 beds and is accredited by the International Joint Commission. The KAAUH is located in the southern area of Princess Noura University (PNU) Campus and it is a 300-bed teaching hospital serving the PNU faculty. The study was approved by the Institutional Review Board of both centers (reference numbers HP-01-R-079 and H-01-R-059 for PSMMC and KAAUH, respectively; ClinicalTrials.gov identifier: NCT04031859).
All patients who were scheduled for elective TKR surgery in the period between October 2018 and July 2019 in both medical centers were eligible for the study. After admittance, those who signed the informed consent form and met the inclusion and exclusion criteria were included in the study. One group (A) was designated as the experimental group. The VTE risk stratification tool that was designed by the first author (see Appendix) was applied to group A in order to choose the tailored extended VTE prophylactic agent. The patients in group A also took part in patient education programs about TKR and its complications (these programs on TKR and the preventive measures that should be taken to avoid TKR complications were given by clinical pharmacist). The other group (B) was designated as the control group. Group B was not assessed by the proposed tool; rather, the Caprini risk assessment tool was used.9 The Caprini tool is the routine hospital protocol employed at the two centers for choosing the VTE prophylactic agent and is a widely used, standard, validated tool. The patients in group B did not participate in any educational programs on TKR; rather, they were given the usual counseling tips regarding possible post-surgery risks. All the patients in group B were categorized as high risk according to the Caprini total risk score. Therefore, each of the patients in group B was prescribed an anticoagulant either orally (rivaroxaban) or subcutaneously (enoxaparin) at a dosage and for a period of time determined by the surgeon according to his/her experience. For group A, the educational programs were done for each patient individually, starting from patients’ admission day, through hospital stay, and during all the follow up visits. Patients were educated on the preventive measures, the exercises that should be done on regular basis to prevent blood clots, and the best way in which VTE prophylactic medications are administered. The authors have recommended the Knee replacement guide of North Bristol, through the educational programs.10
Both groups of patients were followed for 35 days post operation, during which time all VTE or bleeding events were recorded by data collectors using a prospective data collection sheet. In addition, the HAS-BLED score was used to assess the bleeding risk factor for each patient.11
In order to allocate the participants to group A or B, randomization of the study sample was done using random permuted blocks, and a randomization sequence was created by an independent physician using Microsoft Excel version 10 with a 1:1 allocation using random block sizes of 6, 8, 10 or 20. The independent physician provided the data collectors (clinical pharmacist and physicians) with a sealed envelope containing details of the group to which each participant had been allocated. In studies of this type, the best practice is to perform a double-blind randomization. However, in this study, only the participants (patients) were blinded to their group. The data collectors (clinical pharmacist and physicians) were non-blinded, meaning that they were aware of the group to which each participant had been allocated. No changes were made to the trial method or to the outcomes after trial commencement. Moreover, due to the high cost of the adjudication committee we could not use it; because this research was not funded by any institution.
The inclusion criteria for participation in this study were as follows: Male or female patients scheduled for elective TKR surgery (primary only), signed informed consent form, and aged older than 18 years. The exclusion criteria were as follows: Patients receiving anticoagulant treatment, patients with a history of DVT or pulmonary embolism (PE), patients with renal or hepatic failure (where renal failure was defined as end-stage kidney disease (on dialysis) and hepatic failure was defined as complete liver cirrhosis), patients who were pregnant, and patients who were scheduled to have revision surgeries.
Design of the proposed VTE risk stratification protocol
The author designed the proposed VTE risk stratification protocol specifically for TKR patients. In this protocol or tool, the VTE risk factors are divided into two types: patient-specific risk factors and surgery-specific risk factors. The following steps explain how the tool is used and the rationale behind the scoring of the various risk factors:
First step: Identification of patient-specific VTE risk factors
The patient-specific VTE risk factors in the proposed tool are the same as those that are evaluated by the 2005 Caprini risk assessment tool.9 This is because several studies have proven the superiority of the Caprini tool over other tools.12 Therefore, the first step in the VTE risk stratification protocol actually involves using the Caprini tool to calculate the total VTE risk score for each patient, according to the points scored by the patient for each patient-specific risk factor in the Caprini assessment.
Second step: Identification of surgery-specific VTE risk factors
In the second step the surgery-specific VTE risk factors are calculated. These risk factors are length of stay (LOS), operating time, and type of anesthesia. These risk factors were selected due to their positive association with the incidence of VTE events as reported in the literature. First, with regards to LOS, according to Zhang et al. (2018) a hospital stay of longer than 3 days is considered a prolonged stay that will increase post-surgical complications.13 On the other hand, Afshari et al. (2018), considered that day surgery and fast-track surgery are low-risk procedures, where they defined day surgery (or ambulatory surgery) as “a surgical procedure for which the patient is released from the hospital on the same day as surgery or admitted and discharged within 24 h” (p. 78) and fast-track as “surgery after which patients are mobilized within hours after the operation and fully mobilized no later than on the day after surgery, with discharge no later than the fifth day” (p. 78). Therefore, a LOS of more than 5 days will increase VTE risk.14 Hence this risk factor wasgiven a score of 2. As for operating time, Duchman et al. (2017) stated that an operative time >120 minutes is associated with increased short-term morbidity and mortality after primary total joint replacement.15 Therefore, this risk factor was given a score of 1. As regards type of anesthesia, a meta-analysis byHu et al. (2009) revealed that regional anesthesia seems to improve the outcomes of patients who have undergone total hip or knee replacement by reducing the operating time, the need for transfusion, and the incidence of thromboembolic disease.16 Moreover, compared with general anesthesia, spinal anesthesia can reduce postoperative pain (which helps with early ambulation), morphine consumption, nausea and vomiting.17 Accordingly,general anesthesia was given a score of 2 as a surgery-specific VTE risk factor due to its multiple effects on VTE events, duration of surgery, and pain management.
Third step: Calculation of the total VTE risk for patient group stratification
In this step, the total score for the patient-specific VTE risk factors and the total score for the surgery-specific risk factors for each patient are summed to arrive at a total VTE risk factor score for each patient. Then, based on their score, the patients are categorized into one of three groups: low high risk, moderate high risk, and very high risk. To generate the cutoff points for these three groups, the author referred to the cutoff points employed by a standard tool, namely, the 2005 Caprini tool. According to the Caprini risk assessment tool, a total risk score of 1-2 is considered to represent a low level of risk. Therefore, the author selected a cutoff point of 7 for the low high riskgroup in the proposed risk stratification tool, since TKR surgery has a 5 points score, add to this 2 points for other VTE risk (which represent the minimal risk according to Caprini), so it’s a low risk added above the surgery risk, by this it will be considered as low high risk group in the proposed risk stratification tool. On the other hand, a total VTE risk score of 3-4 according to the Caprini tool is considered to denote a moderate level of risk.Hence, 3-4 points were added to the basic 5 points for surgery-specific risks, resulting in a range of 8-9 points for the moderate high riskgroup in the proposed risk stratification tool. Finally, 5 or more added VTE risk points is considered to indicate a very high risk group according the Caprini approach. Therefore, the author decided that a score of 10 or more should denote a very high risk level in the proposed risk stratification tool.
Fourth step: Prescription of the extended VTE prophylaxis based on patientgroup
Based on the results of the VTE risk stratification, the physician was able to select the appropriate extended VTE prophylaxis to administer upon discharge; during hospitalization the patient was prescribed any recommended anticoagulant according to the American College of Chest Physicians guidelines, but upon discharge the extended VTE prophylaxis choice depended on the patient’s risk group.18 If the patient was categorized as low high risk, they were prescribed aspirin as the extended VTE prophylaxis. On the other hand, if their score puts them in moderate high risk category, they were prescribed an oral anticoagulant. Finally, if the patient was categorized as very high risk, they were prescribed a parenteral or oral anticoagulant, depending on the patient’s preference, but the parenteral anticoagulant was reserved for the very high risk group only. Please see the online appendix for dose and duration of each prophylaxis.
Statistical analysis
In this study, the primary outcome was symptomatic VTE events (DVT or PE) within 35 days post TKR surgery. The secondary outcomes were bleeding (minor or major bleeding), surgical site infection, sudden death and readmission within 35 days post TKR surgery. These outcomes were assessed during the hospital stay as well as during the follow-up visits. Major bleeding is defined as bleeding severe enough to require significant medical intervention, such as transfusions or surgery, or those results in serious morbidity or mortality. Minor bleeding is any mild bleeding that does not match the criteria of major bleeding.
It has been reported that the symptomatic VTE rate during the first 3 months post orthopedic surgery is within the range of 1.3% to 10%, while the cumulative incidence of VTE within 90 days of surgery is 3.29%.19,20 A recent study that was done in Saudi Arabia stated that the incidence of symptomatic VTE is 1.9%.21 As the context of this study is also Saudi Arabia, the author used the estimated proportion in the previous Saudi study to calculate the sample size. The formula used to calculate the sample size was based on an estimation of the proportion of patients who were expected to experience symptomatic VTE outcome as follows:
Sample size = 3.84 x p(1-p)/(precision)2
at a 95% confidence interval, where p =estimated proportion (cumulative incidence) =1.9% and precision =0.05. Accordingly, the minimum sample size was calculated as 237. As the sample size for this study was 242, the minimum sample size criterion was achieved.
At the end of the 35-day follow-up, the collected data were analyzed using the Statistical Package for the Social Sciences version 20 at a precision of 0.05 and a confidence interval of 95%. Descriptive analysis was used to describe the characteristics of the sample. The chi-square test and analysis of variance (ANOVA) were used to identify any significant differences between thegroups: ANOVA was used to test for differences between the continuous variables and to compare means, while the chi-square test was conducted to examine the discrete variables (frequencies).
RESULTS
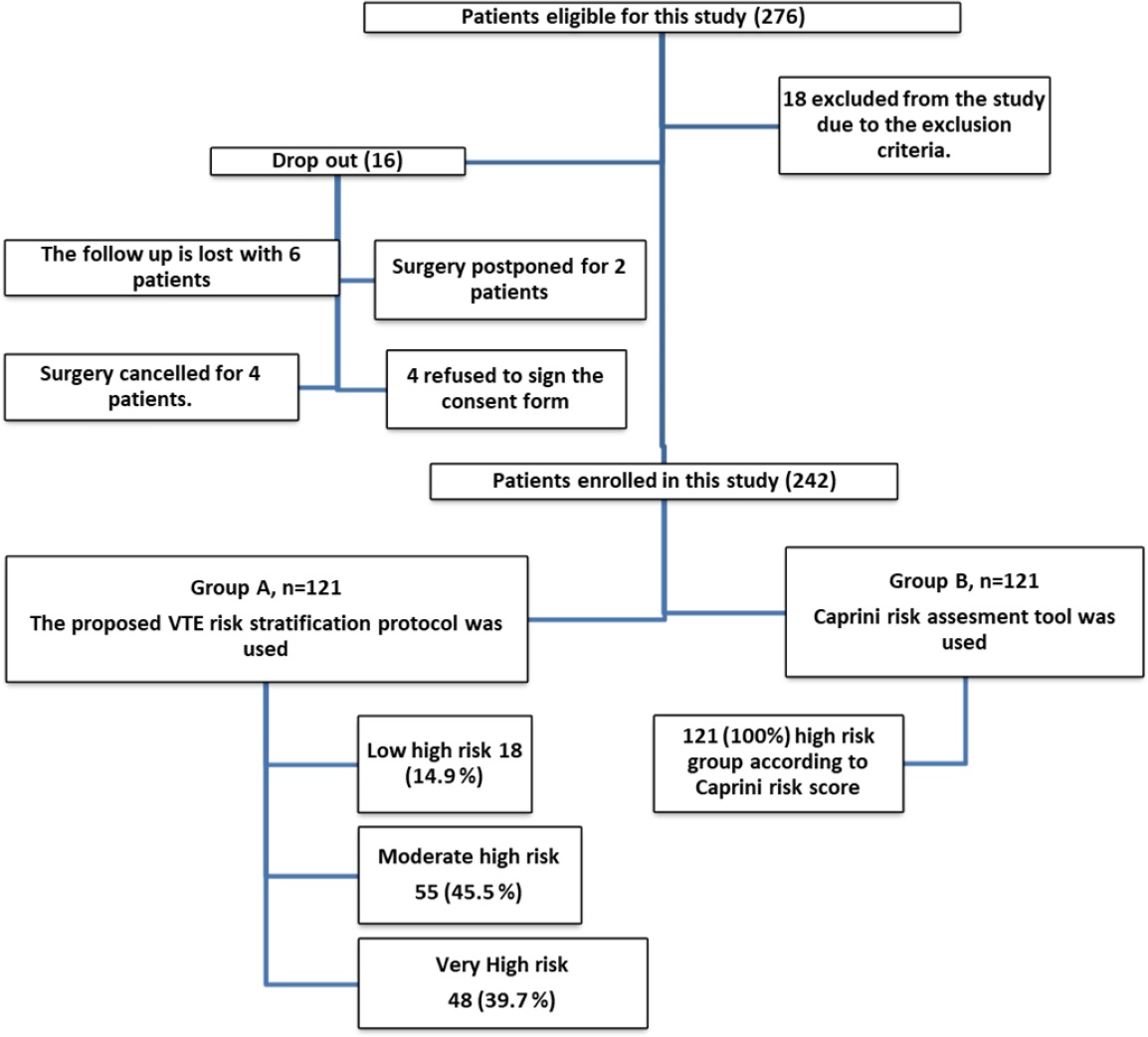
A total of 276 patients were eligible for this study. However, 18 patients were excluded after applying the inclusion and exclusion criteria and four declined to participate, leaving 254. A further 12 patients were dropped from the study for the following reasons: Six were lost to follow-up, surgery was postponed for two patients due to unstable vital signs, and surgery was canceled for four patients for different reasons. The remaining 242 patients signed the informed consent form, indicating their agreement to participate in the study.. The patients in group A were subdivided into three groups according to the level of VTE risk (Figure 1). Group B was designated as the control group. The risk stratification tool was not applied to this group; rather, they were evaluated by using the Caprini (2005) risk assessment tool.
The patients’ demographic characteristics are shown in Table 1. The mean age of all the participants was 65.86 (SD 8.96) years and most of them were female (137/242, 56.6%). The mean body mass index of the study sample was 32.46 (SD 5.51). From a comparison of the characteristics of the experimental group (A) and the control group (B), there were no significant differences between groups in terms of age, gender, BMI, or lifestyle. The main difference found between the two groups was in respect of the surgery type. For unilateral TKR, group B was higher than group A with 81 (33.5%) vs. 52 (21.5%) patients, respectively. As regards bilateral TKR, group A was higher than group B with 69 (28.5%) vs. 40 (16.5%) patients, respectively, at a significant p-value < 0.05. As for the comorbidities and medications of the two groups, as shown in Table 1, there were no significant differences in either medical illnesses or medications between groups A and B. All patients were prescribed the following four types of medication as preventive measures and as prophylaxis during their hospital stay: antibiotics, proton pump inhibitors or H2 blockers, VTE prophylaxis and antiemetic, in addition to analgesics post operation.
Table 1. Demographic data, medical illness and medication comparisons
| Demographics/ Clinical data | Group A N=121 (50.0%) | Group B N=121 (50.0%) | All N=242 | p-value |
|---|---|---|---|---|
| Age Mean (SD) | 65.23 (9.4) | 6.49 (8.5) | 65.86 (8.67) | 0.279 |
| Gender Male (N,%) | 54 (44.6%) | 51 (42.1%) | 105 (43.4%) | 0.697 |
| BMI Mean (SD) | 32.8 (5.89) | 32.1 (5.10) | 32.46 (5.51) | 0.318 |
| Lifestyle*(N,%) | ||||
| Restricted | 58 (47.9%) | 63 (52.1%) | 121 (50.0%) | 0.522 |
| Normally Active | 63 (52.1%) | 58 (47.9%) | 121 (50.0%) | |
| Highly Active | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Type of surgery(N,%) | <0.001 | |||
| Unilateral TKR | 52 (43.0%) | 81 (66.9%) | 133 (55.0%) | |
| Diseases (N,%) | ||||
| Rheumatoid arthritis (RA) | 7 (5.8%) | 4 (3.3%) | 11 (4.5%) | 0.355 |
| Dyslipidemia | 29 (24.0%) | 33 (27.3%) | 62 (25.6%) | 0.556 |
| Osteoarthritis OA | 114 (94.2%) | 117 (96.7%) | 231 (95.5%) | 0.355 |
| Benign prostatic hyperplasia (BPH) | 5 (4.1%) | 3 (2.5%) | 8 (3.3%) | 0.472 |
| Asthma | 5 (4.1%) | 4 (3.3%) | 9 (3.7%) | 0.734 |
| Diabetes mellitus (DM) | 69 (57.0%) | 71 (58.7%) | 140 (57.9%) | 0.795 |
| Chronic kidney disease (CKD) | 0 (0.0%) | 2 (1.7%) | 2 (0.8%) | 0.156 |
| Hypertension (HTN) | 76 (62.8%) | 88 (72.7%) | 164 (67.8%) | 0.099 |
| Ischemic heart disease (IHD) | 13 (10.7%) | 14 (11.6%) | 27 (11.2%) | 0.838 |
| Gout | 2 (0.8%) | 2 (0.8%) | 4 (1.7%) | 1.000 |
| Hypothyroidism | 27 (22.3%) | 26 (21.5%) | 53 (21.9%) | 0.876 |
| Medications | ||||
| H2 blocker (Famotidine or Ranitidine) | 25 (20.7%) | 19 (15.7%) | 44 (18.2%) | 0.317 |
| Analgesic | 121 (100.0%) | 121 (100.0%) | 242 (100.0%) | - |
| A. B | 121 (100.0%) | 121 (100.0%) | 242 (100.0%) | - |
| VTE-Prophylaxis during hospital stay | 121 (100.0%) | 121 (100.0%) | 242 (100.0%) | - |
| Diabetic medication | 69 (57.0%) | 71 (58.7%) | 140 (57.9%) | 0.795 |
| Hypertension medication | 77 (63.6%) | 87 (71.9%) | 164 (67.8%) | 0.169 |
| IHD-Medication | 13 (10.7%) | 14 (11.6%) | 27 (11.2%) | 0.838 |
| Gout medication | 2 (0.8%) | 2 (0.8%) | 4 (1.7%) | 1.000 |
| Proton pump inhibitors (PPIs) | 86 (71.1%) | 75 (62.0%) | 161 (66.5%) | 0.134 |
| Antiemetic | 121 (100.0%) | 121 (100.0%) | 242 (100.0%) | - |
| Levothyroxine | 27 (22.3%) | 24 (19.8%) | 51 (21.1%) | 0.636 |
| Statins | 26 (21.5%) | 31 (25.6%) | 57 (23.6%) | 0.449 |
| Antiplatelet | 7 (5.8%) | 9 (7.4%) | 16 (6.6%) | 0.605 |
AB=antibiotics, BMI= Body Mass Index, CKD = chronic kidney disease, DM = diabetes mellitus, HTN = Hypertension, IHD = ischemic heart disease, N= number or frequency of patients, OA = osteoarthritis, PPIs= Proton pump inhibitors, RA = rheumatoid arthritis, SD = standard deviation, TKR = total knee replacement, VTE = venous thromboembolism. To test the difference between continuous variables, compare means ANOVA test was used. Chi square was conducted to test discrete variables (frequencies).
*Lifestyle: restricted means always sitting, normal means everyday life activity and highly active means exercising on daily basis.
Table 2 shows the hospitalization details for all the participants from admission to discharge. It can be seen that there were no significant differences between the control group (B) and the experimental group (A). The mean duration of hospital stay was 5.68 (SD 1.32) days for all participants. All the artificial implemented knees were the cemented type. The mean duration of surgery was 2.15 (SD 0.618) hours. Regional anesthesia was used for the majority of participants (79.8%), while general anesthesia was given to 20.2%. As regards post-operation pain, 70.2% of the participants reported severe pain 24 hours post operation, 78.5% of participants were prescribed a strong opioid. As an anticoagulation treatment, 96.3% of participants were prescribed Enoxaparin before surgery and 97.1% were prescribed Enoxaparin after surgery. Upon discharge, 52.9% of the participants were prescribed Rivaroxaban (10 mg daily) as the extended VTE prophylaxis, without any significant differences between the two groups. The majority of the participants could not walk on day 0 which is defined as ‘hours post operation’ (98.8%) or on day 1 post operation (93.0%). The mean number of days needed for all patients to start walking post operation was 2.50 (SD 1.08) days, whereas they needed 4.13 (SD 1.5) days to achieve full mobilization post operation. A comparison of VTE and bleeding risk factors are shown in Table 2, according to the HAS-BLED score, the numbers shown in Table 2 represent the number of patients classified as high risk for major bleeding
Table 2. Surgical procedure, treatment, recovery measures, and risk factors
| Clinical data | Group A 121 (50.0%) | Group B 121 (50.0%) | All (N,%) | p-value |
|---|---|---|---|---|
| Duration of hospital stay; Mean (SD) | 5.58 (1.53) | 5.79 (1.08) | 5.68 (1.32) | 0.227 |
| Type of metal implants; (N,%) | - | |||
| Cemented | 121 (100.0%) | 121 (100.0%) | 242 (100.0%) | |
| Cementless | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Others | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Length of surgery; Mean (SD) | 2.08 (0.550) | 2.22 (0.674) | 2.15 (0.618) | 0.088 |
| Type of Anesthesia; (N,%) | 0.426 | |||
| Regional | 99 (81.8%) | 94 (77.7%) | 193 (79.8%) | |
| Pain score; (N,%) | ||||
| Mild pain | 7 (5.8%) | 13 (10.7%) | 20 (8.3%) | |
| Moderate pain | 30 (24.8%) | 22 (18.2%) | 52 (21.5%) | |
| Severe pain | 84 (69.4%) | 86 (71.1%) | 170 (70.2%) | |
| Type of analgesia;(N,%) | 0.947 | |||
| No analgesia | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Non-opioid | 3 (1.3%) | 3 (1.3%) | 6 (2.5%) | |
| Weak opioid | 22 (18.2%) | 24 (19.8%) | 46 (19.0%) | |
| Strong opioid | 96 (79.3%) | 94 (77.7%) | 190 (78.5%) | |
| VTE-Prophylaxis pre-operation; (N,%) | 0.498 | |||
| NON* | 1 (0.4%) | 2 (0.8%) | 3 (1.2%) | |
| Enoxaparin | 118 (97.5%) | 115 (95.0%) | 233 (96.3%) | |
| UFH | 1 (0.4%) | 3 (1.3%) | 4 (1.7%) | |
| Rivaroxaban | 1 (0.4%) | 0 (0.0%) | 1 (0.4%) | |
| Aspirin (N,%) | 0 (0.0%) | 1 (0.4%) | 1 (0.4%) | |
| VTE-Prophylaxis Post-operation; (N,%) | 0.564 | |||
| NON | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Enoxaparin | 119 (98.3%) | 116 (95.9%) | 235 (97.1%) | |
| UFH | 1 (0.8%) | 3 (2.5%) | 4 (1.7%) | |
| Rivaroxaban | 1 (0.8%) | 1 (0.8%) | 2 (0.8%) | |
| Aspirin | 0 (0.0%) | 1 (0.8%) | 1 (0.4%) | |
| Extended VTE after discharge | 0.093 | |||
| Enoxaparin 30mg | 0 (0.0%) | 1 (0.8%) | 1 (0.4%) | |
| Enoxaparin 40mg | 41 (33.9%) | 43 (35.5%) | 84 (34.7%) | |
| Rivaroxaban 10mg | 59 (48.8%) | 69 (57.0%) | 128 (52.9%) | |
| Rivaroxaban 20mg | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Aspirin 160mg | 20 (16.5%) | 8 (6.6%) | 28 (11.6%) | |
| Aspirin 325mg | 1 (0.8%) | 0 (0.0%) | 1 (0.4%) | |
| Mobility within hours post operation (D0) (N,%) | 0 (0.0%) | 3 (2.5%) | 3 (1.2%) | 0.081 |
| Fully mobilized no later than D1 (N,%) | 9 (7.4%) | 8 (6.6%) | 17 (7.0%) | 0.801 |
| Day for start walking post operation; Mean (SD) | 2.51 (1.10) | 2.49 (1.06) | 2.50 (1.08) | 0.859 |
| Day of achieving Fully mobilization post operation; mean (SD) | 4.06 (1.7) | 4.20 (1.3) | 4.13 (1.5) | 0.476 |
| VTE risk factors other than the surgery; (N,%) | 0.386 | |||
| Weak risk factors | 41 (33.9%) | 49 (40.5%) | 90 (37.2%) | |
| Moderate risk factors | 77 (63.6%) | 67 (55.4%) | 144 (59.5%) | |
| Strong risk factors | 3 (1.3%) | 5 (2.0%) | 8 (3.3%) | |
| Caprini Score; Mean (SD) | 7.84 (0.89) | 7.83 (0.91) | 7.83 (0.90) | 0.886 |
| Sheet Score (Group A only); Mean (SD) | 9.62 (2.01) | - | - | - |
| Sheet category (Group A only); (N,%) | - | |||
| Low high risk | 18 (14.9%) | - | - | |
| Moderate high risk | 55 (45.5%) | - | - | |
| Very High risk | 48 (39.7%) | - | - | |
| Risk for bleeding; (N,%) | 4 (3.3%) | 7 (5.8%) | 11 (4.5%) | 0.355 |
D0= same operation day, D1= after 24 hours post operation, SD=standard deviation, UFH= unfractionated heparin, VTE= venous thromboembolism. To test the difference between continuous variables, compare means ANOVA test was used. Chi square was conducted to test discrete variables (frequencies).
*NON: indicates that no VTE prophylaxis was prescribed before surgery.
In the experimental group (A), most patients were classified as moderate high risk (45.5%, 55/121), while 39.7% (48/121) were classified as very high risk and 14.9% (18/121) were classified as low high risk.
In this study, both the experimental (A) and control group (B) were followed up for 35 days post operation. During this follow-up period all complications were recorded and summarized, as shown in Table 3. A total of 15/242 participants (6.2%) experienced DVT symptoms, while PE symptoms were seen in one case 1/242 (0.4%). In contrast, diagnosis using Doppler ultrasound was confirmed for DVT in 12/242 (5.0%) patients but there were no confirmed PE cases. Among the confirmed DVT cases, one was in group A (1/121, 0.8%) and the rest were in group B (11/121, 9.1%), with a significant difference between the two groups (p<0.05). All the VTE complications were seen before day 14 post surgery; however, the follow-up was continued up until 35 days post operation. There were no significant differences between the two groups in respect of bleeding, surgical site infection (SSI), or sudden death post TKR surgery; the total bleeding rate was 0.8% (2/242), the total SSI rate was 0.8% (2/242), and sudden death occurred in one case (0.4%, 1/242). The readmission rate for all patients was 2.5% (6/242), all of the patients who were readmitted were from group B with a significant difference between the two groups at a p-value < 0.05. As regards the compliance measure, 98.8% of all patients claimed that they took their VTE prophylaxis medication as prescribed, while 71.0% were able to relate the name, dose, and schedule for their medication. At the first follow-up visit, which was on day 14 post operation, all patients had taken their prophylaxis medication for the past 14 days. After that, some patients completed an extended course of VTE prophylaxis medication for a period of more than 14 days post operation, with an average of 19.91 (SD 7.45) days for all patients. There were significant differences between the two groups in respect of the prophylaxis medications. Firstly, the mean duration for extended VTE prophylaxis postoperatively was 22.70 (SD 7.90) days for group B, which was higher than for group A whose mean duration was 17.12 (SD 5.78) days (p<0.05).
Table 3. Frequency of all complications post surgeries during follow up period
| Clinical data | Group A N=121 (50.0%) | Group B N=121 (50.0%) | All N=242 | p-value |
|---|---|---|---|---|
| Sudden death (N,%) | 0 (0.0%) | 1 (0.4%) | 1 (0.4%) | 0.318 |
| Confirm VTE cases (PE or DVT) (N,%) | 0.007 | |||
| None | 118 (98.3%) | 107 (88.4%) | 225 (93.4%) | |
| Confirmed DVT | 1 (0.8%) | 11 (9.1%) | 12 (5.0%) | |
| Confirmed PE | 0% | 0% | 0% | |
| Not confirmed DVT | 1 (0.8%) | 2 (1.7%) | 3 (1.2%) | |
| PE (N,%) | 0% | 1 (0.8%) | 1 (0.8%) | |
| Bleeding (N,%) | 0.157 | |||
| No | 120 (100.0%) | 119 (98.3%) | 239 (99.2%) | |
| Yes, minor | 0 (0.0%) | 2 (1.7%) | 2 (0.8%) | |
| Yes, major | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Surgical site Infection (N,%) | 0 (0.0%) | 2 (1.7%) | 2 (0.8%) | 0.157 |
| Readmission (N,%) | 0 (0.0%) | 6 (5.0%) | 6 (2.5%) | 0.014 |
| Did you take the VTE prophylactic medication as prescribed for you? | ||||
| Yes (N,%) | 120 (100.0%) | 118 (97.5%) | 238 (98.8%) | 0.083 |
| Can Tell medication name, dose, and schedule | 88 (73.3%) | 83 (68.6%) | 171 (71.0%) | 0.418 |
DVT= Deep vein Thrombosis, PE=Pulmonary embolism, SD=standard deviation, VTE= venous thromboembolism. To test the difference between continuous variables, compare means ANOVA test was used. Chi square was conducted to test discrete variables (frequencies).
*This is patients’ understanding of the medications.
DISCUSSION
To the best of the author’s knowledge, this study is the first to provide an easy, quick VTE risk assessment protocol for TKR patients, by applying a risk stratification protocol; to ensure that each patient is receiving the tailored VTE prophylactic agent. In addition to the new proposed risk stratification protocol, patients educational programs that was done by the clinical pharmacist have added a synergistic effect. Both interventions have proven their efficacy in reducing complications post TKR surgeries, and this agrees with several studies that have proven the effective role of anticoagulation services presented by pharmacists.22 In this study, a debate could emerge about what is exactly behind the findings, what is the real effect of patients’ educational programs or even the novel risk stratification procedure. Actually, these findings should be attributed to the clinical pharmacists’ interventions, whether in patients’ educational programs or VTE prophylaxis recommendations through the usage of the novel risk stratification procedure, as stated by Scrimenti et al (2019), that all clinical pharmacists can provide education and make recommendations, interpret, and adjust VTE prophylaxis medications’ dosing, and this is exactly what is done in this study, when the clinical pharmacist are allowed to intervene, directly the differences in the outcomes will be apparent.23 Caprini risk assessment tool includes general points for all medical and surgical patients, but the novel risk stratification procedure is specific for TKR patients, in which TKR patients can be categorized into low, moderate, or high VTE risk, which is, in turn, will allow to choose the suitable preventive therapy and duration for each patient according to his/her risk category. While in group B all the patients’ level of risk was high according to Caprini risk assessment tool. In this study, the two randomized groups were similar in terms of patient characteristics. The only difference between the two groups was the type of surgery; a higher number of patients in group A had bilateral TKR as compared to patients in group B. In this study, the total symptomatic VTE within 35 days post operation was 4.95% (12/242), which is within the international range for VTE incidence rate. According to the literature, the symptomatic VTE rate during the first 3 months post orthopedic surgery is within the range of 1.3% to 10%.19 According to Murnaghan et al. (2012), who conducted a study on VTE and bleeding events following elective joint replacement surgeries, 12 out of 2342 patients (0.6%) developed DVT, while 16 out of 2342 (0.7%) patients developed PE.24 On the other hand, Loh et al. (2019) reported a rate of symptomatic DVT post TKR of 4.5%.25 Moreover, Murnaghanet al., 2012 concluded that all the early PE complications were seen in TKR surgeries, while late VTE events were seen in Total hip replacement (THR).24 Meanwhile, Fuji et al. (2017) found that the DVT, PE, and bleeding incidence rate by surgery type is 1.3, 0.2, and 1.0% for TKR.26 Thus, in concordance with most studies, in this study, two cases of minor bleeding were seen during follow-up period, with a bleeding rate of 0.8% (2/242). The authors use HAS-BLED to estimate bleeding risk, while this stratification tool has mainly been used in atrial fibrillation populations, still it could be used in patients receiving anticoagulants.27
As regards major bleeding events, the incidence rate was 0% because no cases of major bleeding were seen during the follow-up period. This outcome is comparable to the bleeding rate post TKR reported in the literature, where the major bleeding events rate is 0.04%.24 The zero incidence rate may be due to the need to have a larger sample size to be able to detect major bleeding events. Regarding SSIs, in this study, the overall rate was 0.8% (2/242) for all patients, which is within the estimated international range. The overall rate of infection post orthopedic surgeries has been reported to range from 0.55% up to 1.77% for primary surgeries, while for revision surgeries it is 2.37%.28 In this study, sudden death occurred in one case, with a rate of 0.4% (1/242), and this case had a bilateral TKR under general anesthesia. According to the National Center for Health Statistics in the United States, VTE is responsible for around 100,000 deaths each year in the United States, and 25% of hospital sudden death cases are due to PE.29
In this study, the readmission rate for all patients was 2.5% (6/242), all of whom were from group B with a significant difference between the two groups at a p-value <0.05. This readmission rate is lower than the rate of 4% for 30 days post TKR surgeries reported in the literature.30 For group A in this study, aspirin was one of the VTE prophylaxis choices, which is in line with several studies that have shown that aspirin represents an effective choice post elective TKR or THR.31 Also, in a recent systematic review Mistryet al. (2017) concluded that aspirin is an effective and safe prophylactic agent post elective arthroplasty.32
Lastly, as a study limitation, when considering the above results, it should be noted that this study was a randomized controlled study, and in these types of studies, the best practice is to use double-blind randomization. In this study, only the participants (patients) were blinded to their group, while the data collectors (clinical pharmacist and physicians) were unblinded so that they could administer the appropriate interventions. While it is recognized that this could be a source of experimenter bias, it was necessary to adopt this approach because the data collectors and physicians needed to know to which group each patient belonged in order to be able to follow the relevant protocol in choosing the VTE extended prophylaxis and whether or not to provide the educational programs. Nevertheless, this study met the most important conditions for successful randomization, i.e., “adequate generation of an unpredictable allocation sequence and concealment of that sequence until assignment occurs”.33
CONCLUSIONS
This study was a randomized controlled trial that demonstrated the positive outcomes of using a new proposed VTE risk stratification protocol which provides an easy, quick procedure for patient-specific VTE risk assessment in order to ensure that each patient is given a tailored VTE prophylactic agent. The results showed that the VTE risk stratification protocol, which was administered in conjunction with patient education programs on TKR, can reduce VTE complications and readmission events post TKR surgery.