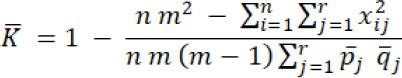INTRODUCTION
An older person is defined by the United Nations as “those aged 60 or 65 years or over.1 Age-related issues that need to be considered are changes in pharmacokinetics and pharmacodynamics, multiple chronic diseases, polypharmacy (more than four medications), cascade prescription, and unnecessary or inappropriate medication.2-5
Polypharmacy is increasing in older persons, and it has been associated with increased risk of adverse drug events, falls, hospitalizations, and emergency admissions.6-12 Polypharmacy is considered part of geriatric syndrome and a predictor of hospitalization, and nursing home placement.13 Additionally, older persons are more likely to be exposed to potentially inappropriate medications (PIMs).6
Deprescribing is defined as a “planned and supervised process of dose reduction or interruption of medication that can cause harm, that does not provide a benefit, or that is considered inappropriate”.5 It is often employed as a strategy to cease medications when one of the following conditions is present: Drugs cause adverse effects, do not have a current indication, are not currently in use, are used irregularly in non-life-threatening conditions, or are used to treat adverse effects of other drugs.14 Deprescribing is a personalized process, because it takes into account the patient's and caregiver's preferences and lifestyle.15 Hence, deprescribing is an essential process but sometimes a challenging task for clinicians.
Recent tools and criteria for deprescribing have been developed to evaluate PIMs.15,16 However, conceptual tools that prompt clinicians to consider, in a logical way, all relevant factors for making prescribing decisions may help to minimize the number of inappropriate drugs. With our proposal, health care professionals may find support on a practical approach to assist clinical decision-making in the deprescribing process.
The Guide to Good Prescribing proposed by the World Health Organization (WHO) establishes throughout its stages the possibility of prescribing only if necessary and deprescribing if necessary. This indicates that prescription/deprescription is a holistic process. Therefore, the aim of this study was to develop and validate a stepwise tool to aid primary health care professionals in the process of deprescribing PIMs in older persons.
METHODS
We planned three consecutive phases: (i) tool design; (ii) expert panel discussion following the Delphi consensus method; and (iii) the inter-rater reliability using Fleiss’ Kappa.
Stage 1: tools design
To identify previously developed deprescribing tools, a literature search in MEDLINE (via PubMed), EMBASE, LILACS, SCIELO was made using the final list of PIM, and terms such as:
((“aged” OR elderly[Title/Abstract] OR older adult[Title/Abstract]) OR frail older adult[Title/Abstract]) AND ((((((“inappropriate prescribing” OR withdrawing treatment[Title/Abstract]) OR over prescribing[Title/Abstract]) OR Inappropriate Drugs[Title/Abstract]) OR deprescription[Title/Abstract]) OR Inappropriate Medications[Title/Abstract]) OR Inappropriate Medicines[Title/Abstract]).
Articles presenting tools, algorithms, and conceptual frameworks to identify PIMs were included from inception to March 2020. The terms were adjusted for each of the databases.
The tool design was structured following content items and domain construction. From the beginning, the proposed tool included four domains (indication, adverse drugs effects, preferences of the patient and their caregiver, assessment and follow up), seven questions organized by the domains and five possible decisions (remove, reduce, switch, continue medication and restart medication). The last step of the tool suggests repeating the process regularly. The tool was developed following the conceptual framework shown in Figure 1.
Stage 2: Delphi consensus method
Pre-consensus
The initial algorithm was proposed by five experts from Spain and Colombia (two family physicians and three clinical pharmacologists) as part of a pre-consensus stage. After constructing a merged proposal with the existing tools, we evaluated the concordance rated for each item, domain and decision included, following an electronic Delphi consensus method.
Rounds
A 2-round electronic Delphi method was conducted to establish consensus. A total of 20 international experts were invited to participate and eighteen accepted to be part of the panel (five geriatricians, two internists, one endocrinologist, three general practitioners, two pharmacologists, three clinical pharmacists, one family physician, and one nurse). Panel members were asked to mark a Likert Scale from 1 to 9 points (1=strongly disagree, 9=strongly agree). It was estimated a rank scale zone (1 to 3; 4 to 6; 7 to 9) to consider strong agreement and thereby to declare consensus.
Round 1 included 16 experts from Colombia, Spain and Argentina who participated by e-mail and were given a 2-week deadline to establish whether they agree with the inclusion of each domain, question (item), and action (decision) category. After the first round, the recommendations given by the experts were accepted, allowing for substantial improvement of the tool. Because the scores of the degree of agreement were distributed along the Likert scale scores, it was necessary to develop a second round.
Round 2 included 18 experts and it was performed to reduce the disagreements from the first round. Both rounds included feedback of the obtained results. Table 1 and Table 2 show the domains (factors), the seven questions, and the actions (decisions) of the proposed tool. The rounds were conducted via e-mail using an electronic survey.
Table 1. Level of agreement in the relevance of each domain, items
| Round 1 (n=16) | Round 2 (n=18) | |||
|---|---|---|---|---|
| Domains (factors) /Questions | Median | Rank | Median | Rank |
| Factor 1. INDICATION | - | - | 9 | 7-9 |
| 1. Is the use of the medication supported by a correct and relevant clinical indication? | 9 | 5-9 | 9 | 9 |
| 2. Does the medication offer a real benefit to the patient according to the prognosis of life? | 8 | 7-9 | 8 | 7-9 |
| 3. Is it the indicated dose appropriate? | 8 | 1-9 | 8 | 8-9 |
| 4. Is there any potentially harmful interaction? | 7 | 3-9 | 7 | 8-9 |
| Factor 2. ADVERSE DRUGS EFFECTS | - | - | 8.5 | 7-9 |
| 5. Is there any risk of adverse drug reaction that exceeds the expected benefits? | 9 | 5-9 | 9 | 9 |
| Factor 3. PREFERENCES OF THE PATIENT AND/OR THEIR CAREGIVER | - | - | 8 | 7-9 |
| 6. Does the patient have a complaint about the use of the medication? | 9 | 1-9 | 9 | 7-9 |
| Factor 4. ASSESSMENT AND FOLLOW UP | - | - | 9 | 7-9 |
| 7. Assess if the evolution of the disease has been exacerbated after the deprescription of the medication | 9 | 3-9 | 9 | 9 |
Domains (-): Not evaluated.
Table 2. Content validity for questions
| Items/domains | Essential | Useful but not essential | Not necessary | CVR | CVR’ | Kappa | Interpretation |
|---|---|---|---|---|---|---|---|
| Factor 1 | 16 | 2 | 0 | 0.77 | 0.88 | 0.69 | Substantial |
| Q1 | 18 | 0 | 0 | 1.00 | 1.00 | 1.00 | Almost perfect |
| Q2 | 15 | 3 | 0 | 0.66 | 0.83 | 0.56 | Moderate |
| Q3 | 18 | 0 | 0 | 1.00 | 1.00 | 1.00 | Almost perfect |
| Q4 | 15 | 3 | 0 | 0.66 | 0.83 | 0.56 | Moderate |
| Factor 2 | 17 | 1 | 0 | 0.88 | 0.94 | 0.83 | Almost perfect |
| Q5 | 18 | 0 | 0 | 1.00 | 1.00 | 1.00 | Almost perfect |
| Factor 3 | 15 | 3 | 0 | 0.66 | 0.83 | 0.56 | Substantial |
| Q6 | 16 | 2 | 0 | 0.77 | 0.88 | 0.69 | Substantial |
| Factor 4 | 17 | 1 | 0 | 0.88 | 0.94 | 0.83 | Almost perfect |
| Q7 | 15 | 3 | 0 | 0.66 | 0.83 | 0.56 | Moderate |
| Total (Ítems) | 115 | 11 | 0 | 5.77 | 6.38 | 0.77 95% CI (0.60-0.93) | Substantial |
| Global CVI | 0.82 | 0.91 | |||||
| Total (domains) | 57 | 15 | 0 | 2.77 | 3.38 | 0.73 95% CI (0.60- 0.86) | Substantial |
| CVI global | 0.69 | 0.84 |
Interpretation criteria for Kappa, using guidelines described by Landis and Koch. Poor= 0.00, slight= 0.01 - 0.20, fair= 0.21 - 0.40, moderate= 0.41 - 0.60, substantial= 0.61 - 0.80 and almost perfect= 0.81 - 1.00. CVR: content validity index. CVR’= content validity ratio. CVI: item-level content validity.
Stage 3: Measurement of interrater reliability
This phase was performed to stablish interrater reliability by measuring the content validity ratio, item-level content validity, and Fleiss’ Kappa of each domain, item, and action.
The inter-rater reliability consisted in determining agreement among raters using a Likert Scale from 1 to 9 points (1=strongly disagree, 9=strongly agree). The level of precision, clarity, and comprehensibility of the tool was acceptable with a minimum of 0.7 (substantial) percent of agreement, using Fleiss’ Kappa statistic.17 The degree of reliability was established by Fleiss’ kappa:
The content validity ratio (CVR) for each item was evaluated using a three-degree range scale (not necessary, useful but not essential, and essential). The minimum acceptance ratio was 0.58, according to Lawshe modified by Tristán.18 Also, the content validity index was used to calculate the global reliability of the tool.
RESULTS
Tool design
The composite tools that were collated from 14 existing tools generated an initial list of 8 questions. Figure 1 shows seven questions included in the proposed tool and these are grouped according to the different domains and actions. The proposal was obtained for a 2-round electronic Delphi method. Each question had two possible answers; yes or no. Supplement 3 displays the algorithm with footnotes for each question in order to extend some considerations for each one.
Delphi consensus method
Round 1: In the first round, the median (砙) values were between 7 and 9 for questions and from 8 to 9 for actions respectively (Table 1). There was no consensus on questions 3, 4, 5, 6, and 7; 1 and 5 had a relative consensus, and 2 was the only one that had agreement in round 1.
The actions (decisions) did not show a definitive consensus in round 1. “Cease” had consensus in this round (砙= 9; 7-9). “Reduce”, “cease/reduce/switch”, “switch” (rank= 1-9) and “continue medication” did not show consensus (rank= 3-9). “Repeat the process regularly” had a relative consensus (rank= 5-9).
As a result of this round, “cease/reduce/switch” had a strong recommendation from experts to be deleted and modified to “cease”. “Reduce” was suggested to change to “adjust” (related to the dose).
Round 2: In the second round, the values of the median were between 7 and 9 for the questions (rank= 7-9), from 8.5 to 9 (rank= 7-9) for domains, and 9 (rank= 7-9) for actions (Table 1). All the proposed changes were accepted. Because the rank values were between 7 and 9 in Likert scale, there was a definite consensus in round 2.
Tool interrater reliability
In round 1, the minimum percentage of agreement for precision, clarity, and comprehensibility of the tool was only achieved for questions 5 and 7. Fleiss’ Kappa was calculated for all items and showed that agreement was not achieved.
In round 2, the minimum percentage of agreement for precision, clarity, and comprehensibility of the tool was achieved for all questions (items), factors (domains) and actions. Fleiss’ Kappa was calculated for all items and it indicated that agreement was accomplished.
All items had a content validity index (CVR) higher than 0.58, meaning all items were accepted. The content validity obtained strength of agreement between moderate, substantial, and almost perfect by the experts, which allows affirming that the tool was sufficient. The global CVI of the instrument was 0.82 and CVI values for items were between 0.66 and 1.00.
Fleiss’ Kappa was 0.77 (95%CI 0.60 to 0.93) for items; 0.73 (95%CI 0.60 to 0.86) for domains, and 0.97 (95%CI 0.90 to 1.00) for actions. According to Landis and Koch, the achieved interrater reliability was between substantial and almost perfect (Table 2 and Table 3).
Table 3. Content validity for actions
| Actions | Essential | Useful but not essential | Not necessary | CVR | CVR’ | Kappa | Interpretation |
|---|---|---|---|---|---|---|---|
| CEASE | 18 | 0 | 0 | 1.00 | 1.00 | 1.00 | Almost perfect |
| ADJUST | 18 | 0 | 0 | 1.00 | 1.00 | 1.00 | Almost perfect |
| SWITCH | 18 | 0 | 0 | 1.00 | 1.00 | 1.00 | Almost perfect |
| CONTINUE | 18 | 0 | 0 | 1.00 | 1.00 | 1.00 | Almost perfect |
| RESTART | 17 | 1 | 0 | 0.88 | 0.94 | 0.83 | Almost perfect |
| TOTAL | 89 | 1 | 0 | 5.13 | 5.06 | 0.97 95%CI (0.90-1.00) | Almost perfect |
| Global CVI | 0.73 | 0.72 |
Interpretation criteria for Kappa, using guidelines described by Landis and Koch. Poor=0.00, slight=0.01- 0.20, fair=0.21- 0.40, moderate=0.41- 0.60, substantial=0.61-0.80 and almost perfect=0.81-1.00. CVR: content validity ratio. CVI: item-level content validity.
DISCUSSION
We propose a tool specifically designed to deprescribe PIMs in older patients. This tool incorporates a step by step systematic approach for identifying, assessing and, withdrawing said medications on an individual basis. Although current tools give us an opportunity to take out a comprehensive list of potentially inappropriate and high-risk medications, further factors of inappropriate prescribing, such as incorrect clinical indication, no benefit, presence of drug interaction (drug-drug, drug-disease, drug-food or drug-herb), adverse drug reactions or any complaint by the patient or caregiver about the use of medications, might be missed.
In the first domain, we included questions that assess the indication of medication. The first question consists in checking if there is a relevant clinical indication for the medication. Thus, this is maybe the most crucial question of the tool because if the answer is “no”, there is no reason to keep the medication, and the action is to cease it. Then, the health provider does not need to follow the next question. It means that the different items are mutually exclusive. The second question evaluates the current benefit of the therapy according to the patient's life prognosis. If the answer is no, the action is to cease the medication. The next item asks if the indicated dose is appropriate. Additionally, the last question in this domain is to establish if there is any potentially harmful interaction. McKean et al. established a tool for identifying and discontinuing unnecessary medications in the inpatient setting, in order to reduce medication burden that only asks about indications, benefits and adverse drug reactions.16
In the second domain, we consider ceasing medications with adverse drug effects that overwhelm the possible benefits. This item intends to identify adverse drug events that might cause negative health outcomes such as falls, hospitalizations, emergency admissions, or conditions caused by medicines.8-12 McKean et al. and Garfinkel et al. also consider this aspect in their proposals.16,19 Adverse drug effects are definitively a critical issue in prescribing/deprescribing process. Hence our proposal also includes this aspect.
In the third domain, we incorporate a question to ask about the preferences of patients or caregivers. If there is any complaint about the use of the medication, the suggestion is to switch to another. Hardy & Hilmer consider that deprescribing should be undertaken as a team approach, not only by involving medical doctor(s), pharmacists and nursing staff, but also patient/caregivers.15 Our proposed tool is the only one that follows an expert validation process and considers the patient's/caregiver's suggestions and complaints as possible and valid reasons for deprescribing.
In the last domain, we include the assessment and follow up of the overall process. If the healthcare professional finds that the patient's conditions have been exacerbated after deprescription, the tool proposes to restart medication. However, it is important to evaluate that additional factors might be the causes of patient's exacerbation. Hence, it is necessary to monitor the patient's condition in order to establish if any other additional factors could potentially explain it. Therefore, we expect that the probability of restarting the prescription is going to be lower because our first two questions are strong. The last step of the process is to repeat the process regularly, considering that patient conditions may change throughout time.
The Delphi method was implemented to assess the validity and reliability of the tool. The domains, questions, and actions were established through a pre-consensus and two discussion rounds with international experts. It is to be noted that consensus methods have been extensively used to validate other well-known tools.20,21 In general, the proposed tool was very well accepted by the experts in the first round. The adjustments made aimed at improving the precision, clarity, and comprehensibility of the tool. Therefore, we proposed to change these measures to cease because if there is a severe adverse drug reaction, the decision would be to withdraw the medication. All the adjustments made in round one allowed for a definitive consensus in round two. Experts noted that our proposal included all the steps performed in real clinical practice.
We acknowledge that the utility of the tool in clinical practice needs to be evaluated, but our expert's validation process confirms that it has all the elements of the real process to make decisions about older persons’ medications. We conducted a robust process for establishing validity and reliability, which means our proposal includes all the domains and questions that the deprescribing process has. Prescribers and health care providers are welcome to use the tool and provide feedback about their perceptions on its utility. Future work will be focused on tool validation by assessing its performance in real clinical settings.
The application of the tool could be limited by the multiplicity of prescribers who evaluate patients (cardiologists, pulmonologists, internists, neurologists, endocrinologists, geriatricians, among others), leading to excessive prescription and making deprescribing difficult. To overcome these difficulties, we suggest all medical specialists to use the tool as a support on decision making about older adult medication. The proposed tool could support medication reconciliation and improve patient health outcomes.
The Canadian D-PRESCRIBE trial is an example of using deprescribing tools for specific medications such as benzodiazepines, z-drugs, first-generation antihistamines, glyburide, and NSAIDs; these tools being used by pharmacists and demonstrating the benefits of deprescribing strategies.22 This study showed that deprescribing promoted a higher number of interruptions of inappropriate prescriptions, when compared to routine care after 6 months, the study's length; meaning that the main outcome evaluated was the reduction of the pharmacological burden caused by these specific medications. However, the results on the benefits emanated from a reduction in hospitalizations, falls, adverse effects, among others, remain to be measured.22
CONCLUSIONS
The proposed tool systematically captures the process of deprescribing performed in clinical practice. It also gives an approximation of the complex pharmacotherapy in older people and reflects the domains, questions, and decisions of deprescribing. The tool includes the preferences and complaints that patients and their caregivers have about medications. This aspect allows the identification of possible reasons for deprescribing that might only be known by patients or caregivers, which often are dismissed or unheard by the practitioners and could potentially allow for a more tailored approach aimed at improving health outcomes. The tool is useful to support the discontinuation of PIMs in older persons and improve their health outcomes.